ARUBA ALOE DEODORANT MEN
EXTRA DRY- aluminum chlorohydrate lotion
Aruba Aloe Balm NV
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
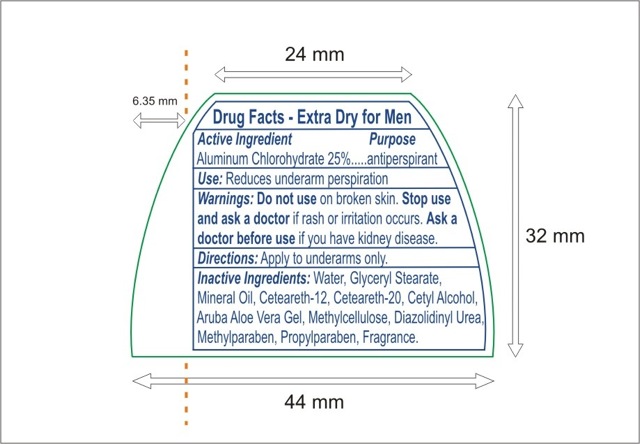
Drug Facts
Warnings: Do not use on broken skin. Stop use and ask a doctor if rash or irritation occurs.
Ask a doctor before use if you have kidney disease.
| ARUBA ALOE DEODORANT MEN
EXTRA DRY
aluminum chlorohydrate lotion |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Aruba Aloe Balm NV (855442273) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aruba Aloe Balm NV | 855442273 | manufacture(53675-132) | |
Revised: 1/2018
Document Id: 635f8720-a870-4ec3-e053-2a91aa0afbe2
Set id: 4a47d4e4-8772-4581-819b-c7200f2d48a1
Version: 3
Effective Time: 20180122
Aruba Aloe Balm NV