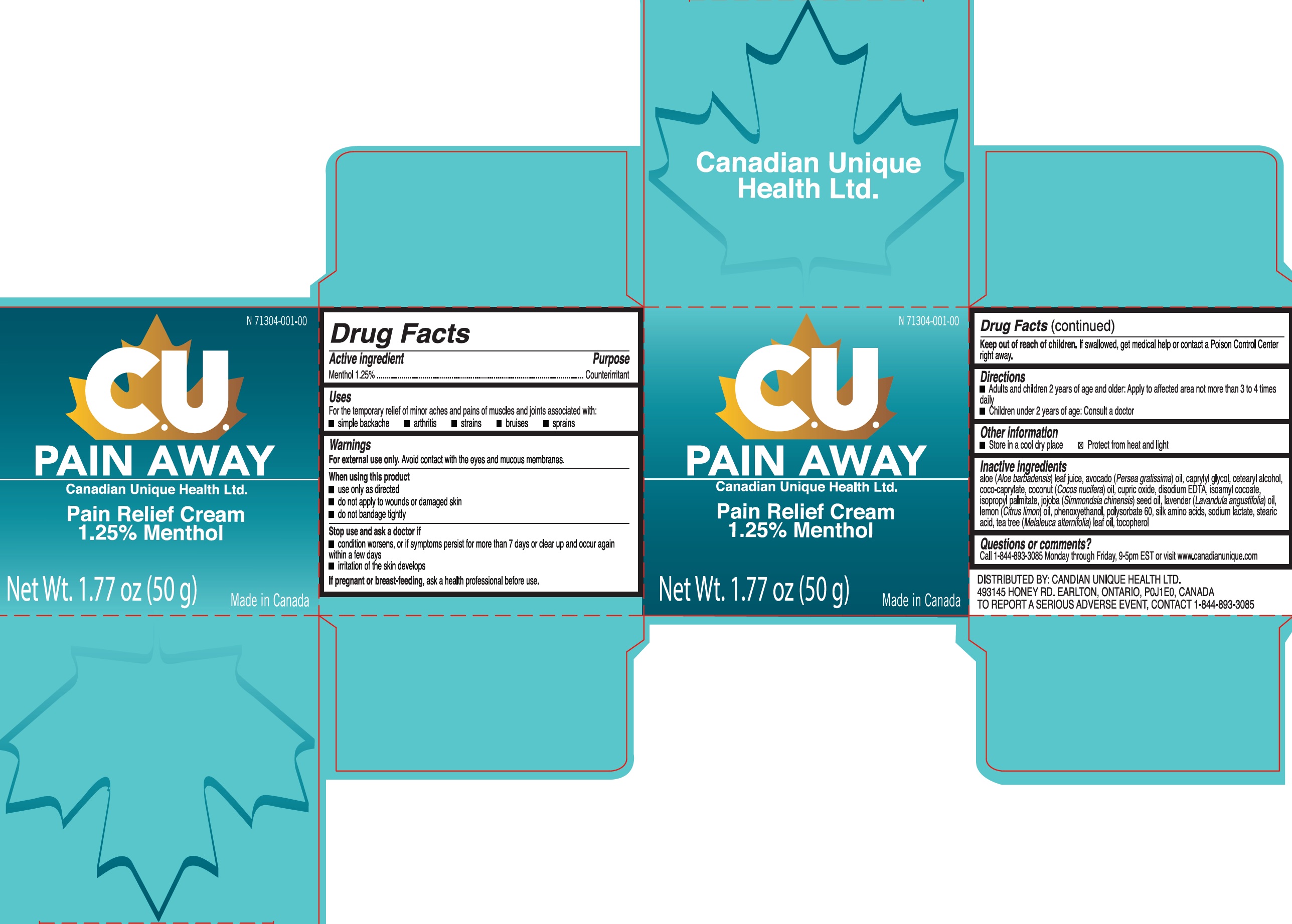
CU PAIN AWAY PAIN RELIEF- menthol cream
Canadian Unique Health Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CU Pain Away Pain Relief
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only. Avoid contact with the eyes and mucous membranes.
When using this product
- use only as directed
- do not apply to wounds or damaged skin
- do not bandage tightly
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
- Children under 2 years of age: Consult a doctor
Inactive ingredients
aloe (Aloe barbadensis) leaf juice, avocado (Persea gratissima) oil, caprylyl glycol, cetearyl alcohol, coco-caprylate, coconut (Cocos nucifera) oil, cupric oxide, disodium EDTA, isoamyl cocoate, isopropyl palmitate, jojoba (Simmondsia chinensis) seed oil, lavender (Lavandula angustifolia) oil, lemon (Citrus limon) oil, phenoxyethanol, polysorbate 60, silk amino acids, sodium lactate, stearic acid, tea tree (Melaleuca alternifolia) leaf oil, tocopherol
| CU PAIN AWAY PAIN RELIEF
menthol cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Canadian Unique Health Ltd. (203413273) |