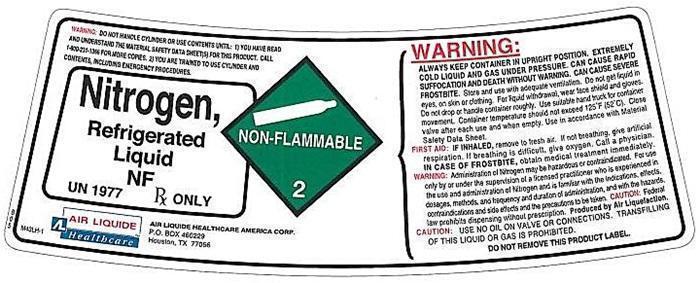
NITROGEN REFRIGERATED LIQUID NF Label
WARNING: DO NOT HANDLE CYLINDER OR USE CONTENTS UNTIL: 1) YOU HAVE READ AND UNDERSTAND THE MATERIAL SAFETY DATA SHEET FOR THIS PRODUCT. CALL 1-800-231-1366 FOR MORE COPIES. 2) YOU ARE TRAINED TO USE CYLINDER AND CONTENTS, INCLUDING EMERGENCY PROCEDURES.
NITROGEN Refrigerated Liquid NF UN 1977 Rx ONLY NON-FLAMMABLE 2
AIR LIQUIDE HEALTHCARE AMERICA CORP.
P.O. BOX 460229
Houston, TX 77056
M43LH-1
WARNING: ALWAYS KEEP CONTAINER IN UPRIGHT POSITION. EXTREMELY COLD LIQUID AND GAS UNDER PRESSURE. CAN CAUSE RAPID SUFFOCATION AND DEATH WITHOUT WARNING. CAN CAUSE SEVERE FROSTBITE.
Store and use with adequate ventilation. Do not get liquid in eyes, on skin or clothing. For liquid withdrawal, wear face shield and gloves. Do not drop or handle container roughly. Use suitable hand truck for container movement. Container temperature should not exceed 125°F (52°C). Close valve after each use and when empty. Use in accordance with Material Safety Data Sheet.
FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Call a physician. IN CASE OF FROSTBITE, obtain medical treatment immediately.
WARNING: Administration of Nitrogen may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of Nitrogen and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects, and the precautions to be taken.
CAUTION: Federal law prohibits dispensing without prescription. Produced by Air Liquefaction.
CAUTION: USE NO OIL ON VALVE OR CONNECTIONS. TRANSFILLING OF THIS LIQUID OR GAS IS PROHIBITED.
DO NOT REMOVE THIS PRODUCT LABEL.