CHILDRENS ALLERGY RELIEF- fexofenadine hydrochloride suspension
HEB
----------
Childrens Allergy Fexofenadine Hydrochloride Oral Suspension
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
• runny nose • itchy, watery eyes
• sneezing • itching of the nose or throat
Warnings
Ask a doctor before use if you have kidney disease. Your doctor should determine if you need a different dose.
When using this product:
• do not take more than directed
• do not take at the same time as aluminum or magnesium antacids
• do not take with fruit juices (see Directions)
Directions
- shake well before using
- use only with enclosed dosing cup
| adults and children 12 years of age and over | take 2 teaspoonfuls (10 mL) every 12 hours; do not take more than 4 teaspoonfuls (20 mL) in 24 hours |
| children 2 to under 12 years of age | take 1 teaspoonful (5 mL) every 12 hours; do not take more than 2 teaspoonfuls (10 mL) in 24 hours |
| children under 2 years of age | ask a doctor |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
• each 5 mL (1 teaspoonful) contains: sodium 18 mg
• safety sealed: do not use if carton, unprinted foil inner seal, or neckband printed with “SEALED FOR YOUR PROTECTION” is opened, torn or missing.
•store between 20° to 25°C (68° to 77°F).
•before using any medication, read all label directions. Keep carton, it contains important information.
Inactive ingredients
artificial raspberry flavor, butylparaben, edetate disodium, poloxamer 407, propylene glycol, propylparaben, purified water, sodium phosphate dibasic, sodium phosphate monobasic, sucrose, titanium dioxide, xanthan gum, xylitol
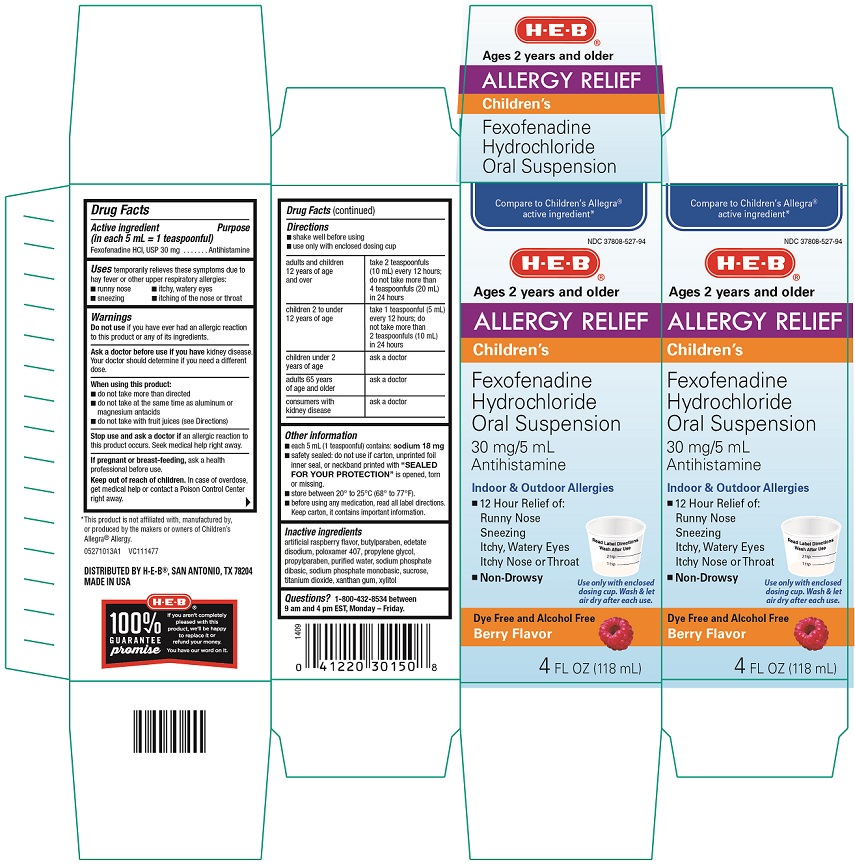
PRINCIPAL DISPLAY PANEL
Compare to Children’s Allegra® active ingredient*
NDC 37808-527-94
HEB®
Ages 2 years and older
Allergy Relief
Children’s
Fexofenadine Hydrochloride Oral Suspension
30 mg/ 5mL
Antihistamine
Indoor and Outdoor Allergies
● 12 Hour Relief of:
Runny nose
Sneezing
Itchy, Watery Eyes
Itchy Nose or Throat
● Non- Drowsy
Use only with enclosed dosing cup. Wash & let air dry after each use.
Dye- Free and Alcohol Free
Berry Flavor
4 fl oz (118 mL)
| CHILDRENS ALLERGY RELIEF
fexofenadine hydrochloride suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - HEB (007924756) |
| Registrant - Teva Pharmaceuticals USA, Inc. (001627975) |