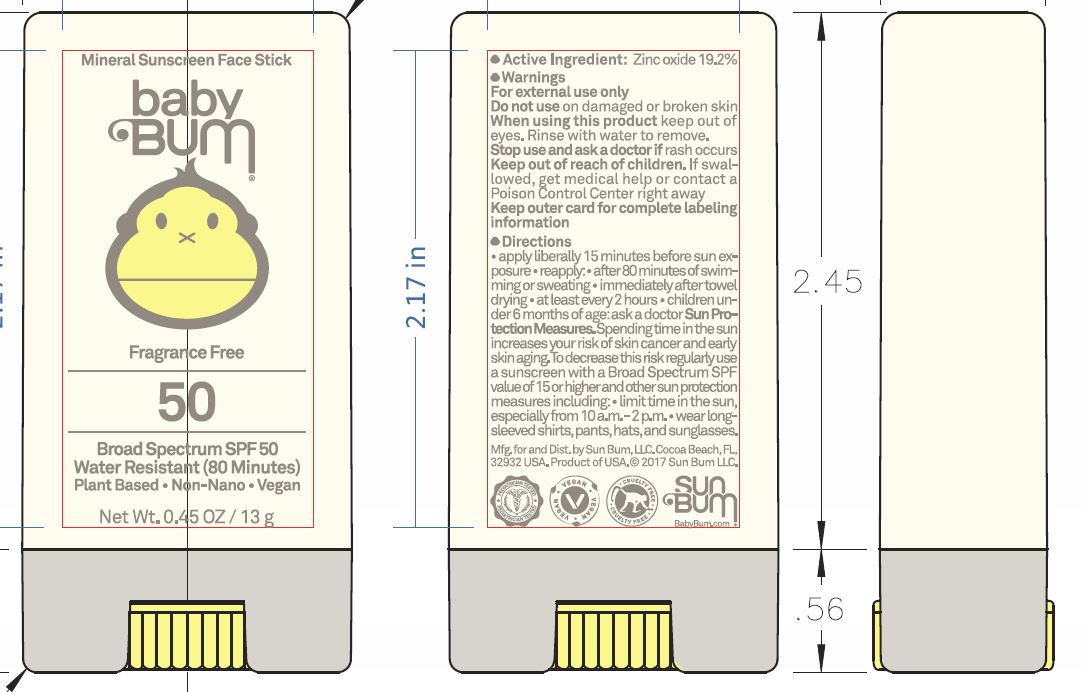
Label: BABY BUM MINERAL SPF50 SUNSCREEN- zinc oxide stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 69039-271-00 - Packager: Sun Bum LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
• apply liberally 15 minutes before sun exposure reapply: • after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours • children under 6 months of age: ask a doctor Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.-2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
cocos nucifera (coconut) oil, persea gratissima (avocado) oil, limnanthes alba (meadowfoam) seed oil, linum usitatissimum (linseed) seed oil, oryza sativa (rice bran) wax, euphorbia cerifera (candelilla) wax, butyloctyl salicylate, copernicia cerifera (carnauba) wax, ozokerite, butyrospermum parkii (shea butter), oryzanol, fragrance, theobroma cacao (cocoa) seed butter, carthamus tinctorius (safflower) seed oil, jojoba esters, tocopherol, bisabolol
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BABY BUM MINERAL SPF50 SUNSCREEN
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69039-271 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 192 mg in 1 g Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) AVOCADO OIL (UNII: 6VNO72PFC1) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) LINSEED OIL (UNII: 84XB4DV00W) RICE BRAN (UNII: R60QEP13IC) CANDELILLA WAX (UNII: WL0328HX19) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CARNAUBA WAX (UNII: R12CBM0EIZ) CERESIN (UNII: Q1LS2UJO3A) SHEA BUTTER (UNII: K49155WL9Y) GAMMA ORYZANOL (UNII: SST9XCL51M) COCOA BUTTER (UNII: 512OYT1CRR) SAFFLOWER OIL (UNII: 65UEH262IS) TOCOPHEROL (UNII: R0ZB2556P8) LEVOMENOL (UNII: 24WE03BX2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69039-271-00 1 in 1 CARTON 06/13/2018 1 13 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/13/2018 Labeler - Sun Bum LLC (028642574)