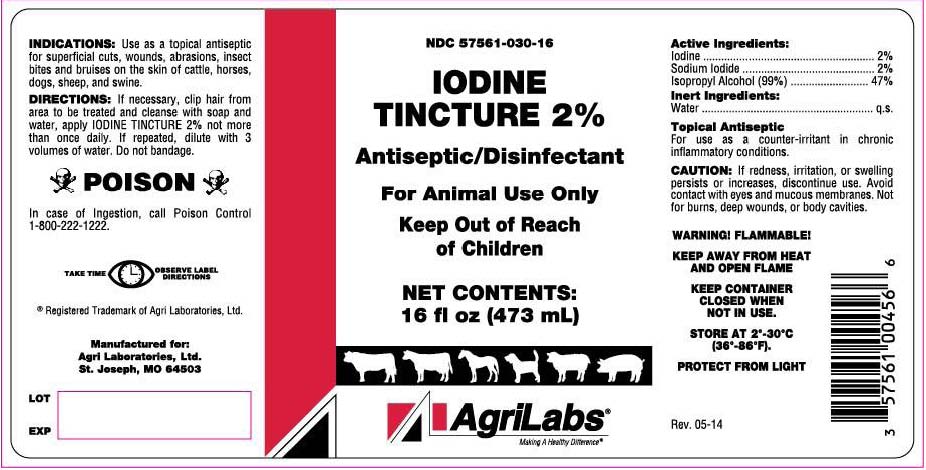
Label: IODINE solution
- NDC Code(s): 57561-030-16, 57561-030-80
- Packager: Agri Laboratories, LTD
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 13, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
Antiseptic/Disinfectant
For animal use only.
Keep out of reach of children.
INDICATIONS
Use as a topical antiseptic for superficial cuts, wounds, abrasions, insect bites and bruises on the skin of cattle, horses, dogs, sheep and swine.
Topical Antiseptic
For use as a counter-irritant in chronic inflammatory conditions.
- DIRECTIONS
- DOSAGE FORMS & STRENGTHS
-
WARNINGS AND PRECAUTIONS
POISON
First Aid: In case of ingestion, call Poison Control 1-800-222-1222
CAUTION
If redness, irritation or swelling persists or increases, discontinue use. Avoid contact with eyes and mucous membranes. Not for burns, deep wounds or body cavities.
WARNING! FLAMMABLE!
KEEP AWAY FROM HEAT AND OPEN FLAME.
KEEP CONTAINER CLOSED WHEN NOT IN USE.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IODINE
iodine solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:57561-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 0.02 mg in 1 mL SODIUM IODIDE (UNII: F5WR8N145C) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 0.02 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.47 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57561-030-16 473 mL in 1 BOTTLE 2 NDC:57561-030-80 3785 mL in 1 JUG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2010 Labeler - Agri Laboratories, LTD (155594450)