ASTHMA- arsenicum iodatum, blatta orientalis, bryonia, carbo vegetabilis, eupatorium perfoliatum, histaminum hydrochloricum, ipecacuanha, lung suis, mercurius corrosivus, natrum sulphuricum, quebracho, sambucus nigra, sticta pulmonaria, urtica urens spray
Liddell Laboratories
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
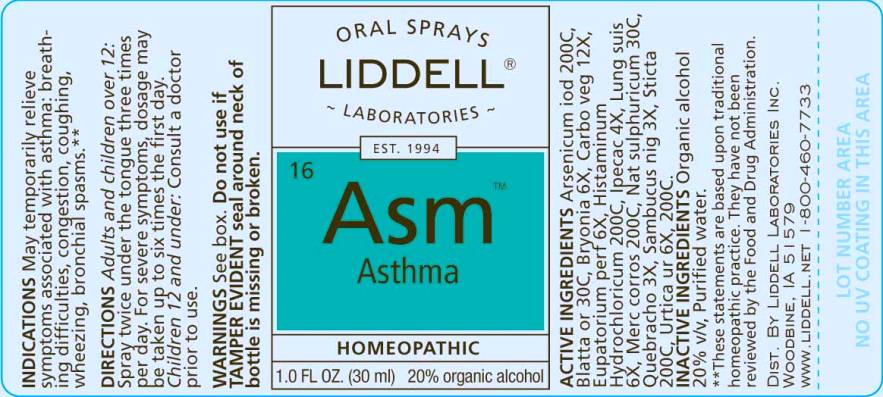
Arsenicum iodatum 200C, Blatta orientalis 30C, Bryonia 6X, Carbo vegetabilis 12X, Eupatorium perfoliatum 6X, Histaminum hydrochloricum 200C, Ipecacuanha 4X, Lung suis 6X, Mercurius corrosivus 200C, Natrum sulphuricum 30C, Quebracho 3X, Sambucus nigra 3X, Sticta pulmonaria 200C, Urtica urens 6X, 200C.
USES:
May temporarily relieve symptoms associated with asthma:
- breathing difficulties,
- congestion,
- coughing,
- wheezing,
- bronchial spasms**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if symptoms persist, worsen or if new symptoms occur.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast-feeding, ask a doctor before using product.
OTHER INFORMATION: Store at room temperature.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
DIRECTIONS:
Adults and children over 12: Spray twice under the tongue 3 times per day. For severe symptoms, dosage may be taken up to six times the first day.
Children 12 and under: Consult a doctor prior to use.
INDICATIONS:
May temporarily relieve symptoms associated with asthma: breathing difficulties, congestion, coughing, wheezing, bronchial spasms **
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
QUESTIONS:
DIST. BY:
LIDDELL LABORATORIES
201 APPLE BLVD.
WOODBINE, IA 51579
WWW.LIDDELL.NET
1-800-460-7733
PACKAGE LABEL DISPLAY:
ORAL SPRAYS
LIDDELL
LABORATORIES
EST 1994
16 Asm
Asthma
May relieve symptoms associated
with asthma: breathing difficulties,
congestion, coughing, wheezing,
bronchial spasms.
HOMEOPATHIC
DOCTOR FORMULATED
Readily absorbed. Safe.
No known side effects. Easy to use.
1.0 FL OZ (30 ml)

| ASTHMA
arsenicum iodatum, blatta orientalis, bryonia, carbo vegetabilis, eupatorium perfoliatum, histaminum hydrochloricum, ipecacuanha, lung suis, mercurius corrosivus, natrum sulphuricum, quebracho, sambucus nigra, sticta pulmonaria, urtica urens spray |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Liddell Laboratories (832264241) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(50845-0112) , api manufacture(50845-0112) , label(50845-0112) , pack(50845-0112) | |