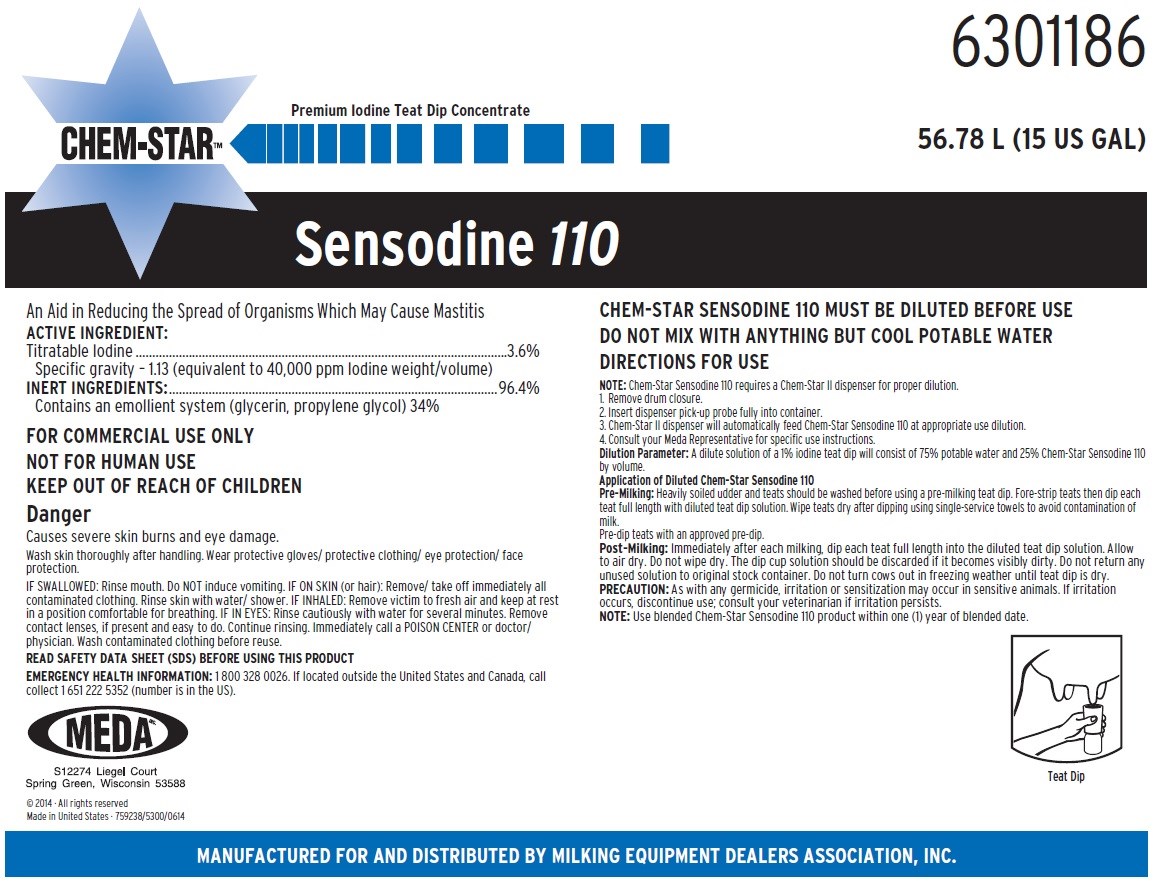
CHEM-STAR SENSODINE 110 - iodine solution
MEDA Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
FOR COMMERCIAL USE ONLY
NOT FOR HUMAN USE
KEEP OUT OF REACH OF CHILDREN
Danger
Causes severe skin burns and eye damage.
Wash skin thoroughly after handling. Wear protective gloves/ protective clothing/ eye protection/ face protection.
CHEM-STAR SENSODINE 110 MUST BE DILUTED BEFORE USE
DO NOT MIX WITH ANYTHING BUT COOL POTABLE WATER
DIRECTIONS FOR USE
NOTE: Chem-Star Sensodine 110 requires a Chem-Star II dispenser for proper dilution.
1. Remove drum closure.
2. Insert dispenser pick-up probe fully into container.
3. Chem-Star II dispenser will automatically feed Chem-Star Sensodine 110 at appropriate use dilution.
4. Consult your Meda Representative for specific use instructions.
Dilution Parameter: A dilute solution of a 1% iodine teat dip will consist of 75% potable water and 25% Chem-Star Sensodine 110 by volume.
Application of Diluted Chem-Star Sensodine 110
Pre-Milking: Heavily soiled udder and teats should be washed before using a pre-milking teat dip. Fore-strip teats then dip each teat full length with diluted teat dip solution. Wipe teats dry after dipping using single-service towels to avoid contamination of milk.
Pre-dip teats with an approved pre-dip.
Post-Milking: Immediately after each milking, dip each teat full length into the diluted teat dip solution. Allow to air dry. Do not wipe dry. The dip cup solution should be discarded if it becomes visibly dirty. Do not return any unused solution to original stock container. Do not turn cows out in freezing weather until teat dip is dry.
PRECAUTION: As with any germicide, irritation or sensitization may occur in sensitive animals. If irritation occurs, discontinue use; consult your veterinarian if irritation persists.
NOTE: Use blended Chem-Star Sensodine 110 product within one (1) year of blended date.
First Aid
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. IF ON SKIN (or hair): Remove/ take off immediately all contaminated clothing. Rinse skin with water/ shower. IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER or doctor/ physician. Wash contaminated clothing before reuse.
READ SAFETY DATA SHEET (SDS) BEFORE USING THIS PRODUCT
EMERGENCY HEALTH INFORMATION: 1 800 328 0026. If located outside the United States and Canada, call collect 1 651 222 5352 (number is in the US).
Principal Display Panel and Representative Label
6301186
Premium Iodine Teat Dip Concentrate
CHEM-STAR™
56.78 L (15 US GAL)
Sensodine 110
An Aid in Reducing the Spread of Organisms Which May Cause Mastitis
ACTIVE INGREDIENT:
Titratable Iodine.................................................................................................................3.6%
Specific gravity – 1.13 (equivalent to 40,000 ppm Iodine weight/volume)
INERT INGREDIENTS:....................................................................................................96.4%
Contains an emollient system (glycerin, propylene glycol) 34%
MEDA
S12274 Liegel Court
Spring Green, Wisconsin 53588
©2014 • All rights reserved
Made in United States · 759238/5300/0614
MANUFACTURED FOR AND DISTRIBUTED BY MILKING EQUIPMENT DEALERS ASSOCIATION, INC.
| CHEM-STAR SENSODINE 110
iodine solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MEDA Inc. (830359597) |