HYDROCORTISONE- hydrocortisone ointment
CMP Pharma, Inc.
----------
HYDROCORTISONE 1% IN ABSORBASE®
Hydrocortisone Ointment USP, 1%
DESCRIPTION
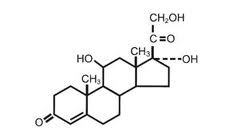
Hydrocortisone 1% in Absorbase® contains 10 mg/g of micronized hydrocortisone USP in a special absorption ointment base. Absorbase® is a water-in-oil emulsion composed of cholesterolized petrolatum and purified water USP. The product will absorb water into the internal emulsion phase, yet form a hydrophobic film on the skin. Hydrocortisone USP is C21H30O5; Pregn-4-ene-3, 20-dione, 11, 17, 21-trihydroxy-, (11 beta)-, Cortisol, and has the structural formula:
Action
Topical corticosteroids are primarily effective as anti-inflammatory, anti-pruritic and vasoconstrictive agents.
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for the treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
INDICATIONS AND USAGE
Topical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
CONTRAINDICATIONS
Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
PRECAUTIONS
General
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients.
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings.
Therefore, patients receiving a large dose of a potent topical steroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids.
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity.
If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
Information for the Patient
Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Patients should be advised not to use this medication for any disorder other than that for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- Patients should report any signs of local adverse reactions, especially under occlusive dressing.
- Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings.
Laboratory Tests
The following tests may be helpful in evaluating the HPA axis suppression:
- Urinary free cortisol test
- ACTH stimulation test
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results.
Pregnancy Category C
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in approximate decreasing order of occurrence:
- Burning
- Itching
- Irritation
- Dryness
- Folliculitis
- Hypertrichosis
- Acneiform eruptions
- Hypopigmentation
- Perioral dermatitis
- Allergic contact dermatitis
- Maceration of the skin
- Secondary infection
- Skin atrophy
- Striae
- Miliaria
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects.
DOSAGE AND ADMINISTRATION
Topical corticosteroids are generally applied to the affected area as a thin film from two to four times daily depending on the severity of the condition.
Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions.
If an infection develops, the use of occlusive dressing should be discontinued and appropriate antimicrobial therapy instituted.
HOW SUPPLIED
Each gram of Hydrocortisone 1% in Absorbase® contains 10 mg of micronized Hydrocortisone USP. Supplied in 1 pound jars (430 g) (NDC 46287-003-16); 4 oz. jars (110 g) (NDC 46287-003-04); and 1 oz. jars (25 g) (NDC 46287-003-01).
Caution
Federal law prohibits dispensing without prescription. For external use only.
PHARMACIST
Water may bleed from this product due to the nature of the water-in-oil emulsion. This separation does not affect the stability of hydrocortisone. The Absorbase® base should be remixed if necessary before dispensing. Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature]
CMP Pharma, Inc.
PO Box 147
Farmville, North Carolina 27828
Revised July 2015
Copyright © CMP Pharma, Inc. 2015
PRINCIPAL DISPLAY PANEL - 430 g Jar Label
NDC 46287-003-16
430 g
Hydrocortisone 1% in Absorbase®
HYDROCORTISONE OINTMENT, USP 1%
Each gram contains 10 mg of micronized Hydrocortisone USP in an aqueous
cholesterolized petrolatum emulsion base.
For External Use Only. Not for Ophthalmic Use.
Store at 20°-25°C (68°-77°F); excursions permitted to
15°-30°C (59°-86°F). [See USP controlled room temperature].
USUAL DOSAGE: See insert labeling.
Dispense in a well-closed container.
Rx Only
LOT:
EXP:
cmp
PHARMA
Farmville, NC 27828

| HYDROCORTISONE
hydrocortisone ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - CMP Pharma, Inc. (005224175) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CMP Pharma, Inc. | 005224175 | MANUFACTURE(46287-003) | |