Label: PYRIDOSTIGMINE BROMIDE tablet
- NDC Code(s): 68382-659-01, 68382-659-05, 68382-659-06, 68382-659-16
- Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
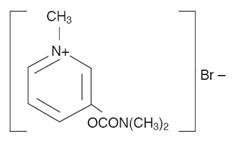
Pyridostigmine bromide tablets, USP are an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:
Pyridostigmine bromide, USP is a white or almost white crystalline, deliquescent powder. It is very soluble in water and in alcohol, slightly soluble in hexane, practically insoluble in ether.
Each pyridostigmine bromide tablet, USP intended for oral administration contains 60 mg of pyridostigmine bromide, USP. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, low substituted hydroxypropyl cellulose, silicon dioxide and stearic acid.
-
CLINICAL PHARMACOLOGY
Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin®#), but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of pyridostigmine bromide or other drugs of this class in the presence of cholinergic crisis or of a refractory or "insensitive" state could have grave consequences. Osserman and Genkins1 indicate that the differential diagnosis of the two types of crisis may require the use of Tensilon®# (edrophonium chloride) as well as clinical judgment. The treatment of the two conditions obviously differs radically. Whereas the presence of myasthenic crisis suggests the need for more intensive anticholinesterase therapy, the diagnosis of cholinergic crisis, according to Osserman and Genkins,1 calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
For detailed information on the management of patients with myasthenia gravis, the physician is referred to one of the excellent reviews such as those by Osserman and Genkins,2 Grob3 or Schwab.4,5
Usage in Pregnancy: The safety of pyridostigmine bromide during pregnancy or lactation in humans has not been established. Therefore, use of pyridostigmine bromide in women who may become pregnant requires weighing the drug's potential benefits against its possible hazards to mother and child.
- PRECAUTIONS
-
ADVERSE REACTIONS
The side effects of pyridostigmine bromide are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
-
DOSAGE AND ADMINISTRATION
Pyridostigmine bromide tablets, USP is available as
Conventional Tablets - each containing 60 mg pyridostigmine bromide.
Dosage: The size and frequency of the dosage must be adjusted to the needs of the individual patient.
Conventional Tablets - The average dose is ten 60 mg tablets, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice.
Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon®# (edrophonium chloride).
-
HOW SUPPLIED
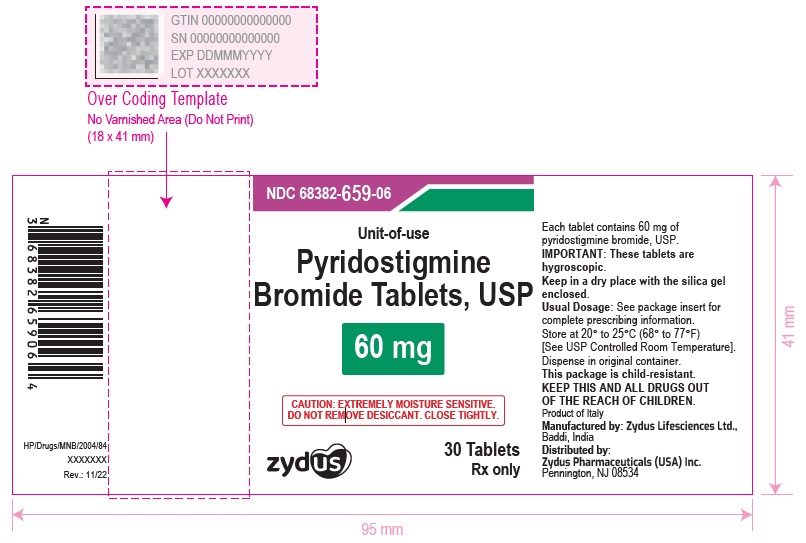
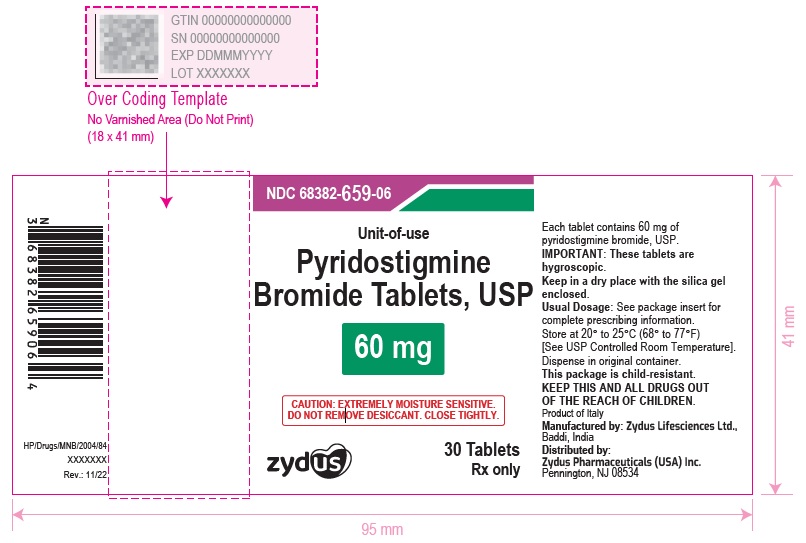
Pyridostigmine Bromide Tablets USP, 60 mg having functional scoring. Pyridostigmine Bromide Tablets USP, 60 mg are white to off-white, round, flat, uncoated tablets with quadrisect breakline on one side and debossed with '659' on the other side and are supplied as follows:
NDC 68382-659-06 in bottles of 30 tablets with child-resistant closure
NDC 68382-659-16 in bottles of 90 tablets with child-resistant closure
NDC 68382-659-01 in bottles of 100 tablets with child-resistant closure
NDC 68382-659-05 in bottles of 500 tablets
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in original container.
IMPORTANT: These tablets are hygroscopic. Keep in a dry place with the silica gel enclosed.
-
REFERENCES
- Osserman KE, Genkins G. Studies in myasthenia gravis: Reduction in mortality rate after crisis. JAMA. Jan 1963; 183:97-101.
- Osserman KE, Genkins G. Studies in myasthenia gravis. NY State J Med. June 1961; 61:2076-2085.
- Grob D. Myasthenia gravis. A review of pathogenesis and treatment. Arch Intern Med. Oct 1961; 108:615-638.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:596-597.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:717-719.
- Cronnelly R, Stanski DR, Miller RD, Sheiner LB. Pyridostigmine kinetics with and without renal function. Clin Pharmacol Ther. 1980; 28:No. 1, 78-81.
- Miller RD. Pharmacodynamics and pharmacokinetics of anticholinesterase. In: Ruegheimer E, Zindler M, ed. Anaesthesiology. (Hamburg, Germany: Congress; Sep 14-21, 1980; 222-223.) (Int Congr. No. 538), Amsterdam, Netherlands: Excerpta Medica; 1981.
- Breyer-Pfaff U, Maier U, Brinkmann AM, Schumm F. Pyridostigmine kinetics in healthy subjects and patients with myasthenia gravis. Clin Pharmacol Ther. 1985;5:495-501.
#Brands mentioned are trademarks of their respective owners.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured by:
Zydus Lifesciences Ltd.,
Baddi, India
Distributed by:
Zydus Pharmaceuticals USA Inc.
Pennington, NJ 08534
Rev.: 11/22
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PYRIDOSTIGMINE BROMIDE
pyridostigmine bromide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68382-659 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOSTIGMINE BROMIDE (UNII: KVI301NA53) (PYRIDOSTIGMINE - UNII:19QM69HH21) PYRIDOSTIGMINE BROMIDE 60 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color WHITE (white to off-white) Score 4 pieces Shape ROUND Size 10mm Flavor Imprint Code 659 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68382-659-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/07/2015 2 NDC:68382-659-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/07/2015 3 NDC:68382-659-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/07/2015 4 NDC:68382-659-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205650 08/07/2015 Labeler - Zydus Pharmaceuticals (USA) Inc. (156861945)