KETEK- telithromycin tablet, film coated
sanofi-aventis U.S. LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KETEK® safely and effectively. See full prescribing information for KETEK.
KETEK (telithromycin) tablets, for oral use Initial U.S. Approval: 2004 WARNING: CONTRAINDICATION IN MYASTHENIA GRAVISSee full prescribing information for complete boxed warningFatal and life-threatening respiratory failure has been reported in patients with myasthenia gravis associated with use of KETEK (4.1) RECENT MAJOR CHANGES
INDICATIONS AND USAGEKETEK is a ketolide antibacterial indicated for the treatment of community-acquired pneumonia of mild to moderate severity. (1) To reduce the development of drug-resistant bacteria and maintain the effectiveness of KETEK and other antibacterial drugs, KETEK should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSTablets: 300 mg and 400 mg (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (2% or greater) are diarrhea, nausea, dizziness, and vomiting (6.1) To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSThe safety and effectiveness under the age of 18 years has not been established. (8.4) See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2015 |
FULL PRESCRIBING INFORMATION
WARNING: CONTRAINDICATION IN MYASTHENIA GRAVIS
There have been reports of fatal and life-threatening respiratory failure in patients with myasthenia gravis associated with the use of KETEK. [see Contraindications (4.1)]
1 INDICATIONS AND USAGE
KETEK is indicated for the treatment of community-acquired pneumonia (of mild to moderate severity) due to Streptococcus pneumoniae, (including multi-drug resistant S. pneumoniae [MDRSP1]), Haemophilus influenzae, Moraxella catarrhalis, Chlamydophila pneumoniae, or Mycoplasma pneumoniae, for patients 18 years or older.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of KETEK and other antibacterial drugs, KETEK should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- 1
- MDRSP, Multi-drug resistant Streptococcus pneumoniae includes isolates known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are isolates resistant to two or more of the following antibacterials: penicillin, 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The dosage of KETEK tablets is 800 mg (2 tablets of 400 mg) taken orally once daily for 7–10 days in patients 18 years or older. KETEK tablets can be administered with or without food.
2.2 Dosage in Patients with Renal and/or Hepatic Impairment
In the presence of severe renal impairment (CLCR less than 30 mL/min), including patients who need dialysis, reduce the dosage of KETEK to 600 mg once daily. In patients undergoing hemodialysis, give KETEK after the dialysis session on dialysis days. [see Clinical Pharmacology (12.3)]
In the presence of severe renal impairment (CLCR less than 30 mL/min), with coexisting hepatic impairment, reduce the dosage of KETEK to 400 mg once daily. Patients with mild or moderate renal impairment (CLCR of 30 mL/min or more) with or without coexisting hepatic impairment do not require a dosage adjustment. No dosage adjustments of KETEK are necessary in patients with hepatic impairment alone. [see Clinical Pharmacology (12.3)]
3 DOSAGE FORMS AND STRENGTHS
KETEK tablets are available in two strengths:
- Tablets: 400 mg supplied as light-orange, oval, film-coated tablets, imprinted "H3647" on one side and "400" on the other side.
- Tablets: 300 mg supplied as light-orange, oval, film-coated tablets, imprinted "38AV" on one side and blank on the other side.
4 CONTRAINDICATIONS
4.1 Myasthenia Gravis
KETEK is contraindicated in patients with myasthenia gravis. Exacerbations of myasthenia gravis have been reported in patients and sometimes occurred within a few hours of the first dose of KETEK. Reports have included fatal and life-threatening acute respiratory failure with a rapid onset and progression.
4.2 Hepatitis
KETEK is contraindicated in patients with previous history of hepatitis and/or jaundice associated with the use of KETEK tablets, or any macrolide antibacterial. [see Warnings and Precautions (5.1)]
4.3 Hypersensitivity
KETEK is contraindicated in patients with a history of hypersensitivity to telithromycin, any components of KETEK tablets, or any macrolide antibacterial.[see Description (11)]
4.4 Cisapride/Pimozide
Concomitant administration of KETEK with cisapride or pimozide is contraindicated because co-administration can lead to life-threatening QT prolongation. [see Warnings and Precautions (5.2); Drug Interactions (7)]
4.5 Colchicine
Concomitant administration of KETEK and colchicine is contraindicated in patients with renal or hepatic impairment due to increased plasma concentration of colchicine leading to life-threatening colchicine toxicity. [see Warnings and Precautions (5.4); Drug Interactions (7)]
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
Acute hepatic failure and severe liver injury, in some cases fatal, have been reported in patients treated with KETEK. These hepatic reactions included fulminant hepatitis and hepatic necrosis leading to liver transplant, and were observed during or immediately after treatment. In some of these cases, liver injury progressed rapidly and occurred after administration of a few doses of KETEK.
Physicians and patients should monitor for the appearance of signs or symptoms of hepatitis, such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, alcoholic stools, liver tenderness, or hepatomegaly. Patients with signs or symptoms of hepatitis must be advised to discontinue KETEK and immediately seek medical evaluation, which should include liver function tests. If clinical hepatitis or transaminase elevations combined with other systemic symptoms occur, KETEK should be permanently discontinued.
KETEK is contraindicated in patients with a previous history of hepatitis and/or jaundice associated with the use of KETEK tablets, or any macrolide antibacterial. [see Contraindications (4.2)]
In addition, less severe hepatic dysfunction associated with increased liver enzymes, hepatitis and in some cases jaundice was reported with the use of KETEK. These events associated with less severe forms of liver toxicity were reversible.
5.2 QTc Prolongation
KETEK can prolong the QTc interval of the electrocardiogram in some patients leading to an increased risk for ventricular arrhythmias, including ventricular tachycardia and torsades de pointes with fatal outcomes. Thus, KETEK should be avoided in patients with congenital prolongation of the QTc interval, and in patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (e.g., quinidine and procainamide) or Class III (e.g., dofetilide) antiarrhythmic agents.
Cases of ventricular arrhythmias (including ventricular tachycardia and torsades de pointes) have been reported post-marketing with KETEK and sometimes occurred within a few hours of the first dose. In clinical trials, no cardiovascular morbidity or mortality attributable to QTc prolongation occurred with KETEK treatment in 4780 patients, including 204 patients having a prolonged QTc at baseline.
5.3 Visual Disturbances and Loss of Consciousness
KETEK may cause visual disturbances particularly in slowing the ability to accommodate and the ability to release accommodation. Visual disturbances, some of them severe, included blurred vision, difficulty focusing, and diplopia. [see Adverse Reactions (6.1)]
There have been post-marketing reports of transient loss of consciousness including some cases associated with vagal syndrome.
Because of potential visual difficulties or loss of consciousness, patients should attempt to minimize activities such as driving a motor vehicle, operating heavy machinery or engaging in other hazardous activities during treatment with KETEK. If patients experience visual disorders or loss of consciousness while taking KETEK, patients should not drive a motor vehicle, operate heavy machinery or engage in other hazardous activities.
5.4 Serious Adverse Reactions with Concomitant Drugs
Serious adverse reactions have been reported in patients taking KETEK concomitantly with CYP3A4 substrates [see Drug Interactions (7)]:
- Colchicine: colchicine toxicity
- Simvastatin, lovastatin, and atorvastatin: rhabdomyolysis
- Calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, amlodipine, diltiazem): hypotension
Colchicine Toxicity
Life-threatening and fatal drug interactions have been reported in patients treated with colchicine and strong CYP3A4 inhibitors. KETEK is a strong CYP3A4 inhibitor and this interaction may occur while using both drugs at their recommended dosages. If co-administration of KETEK and colchicine is necessary in patients with normal renal and hepatic function, reduce the dosage of colchicine. Monitor patients for clinical symptoms of colchicine toxicity. Concomitant administration of KETEK and colchicine is contraindicated in patients with renal or hepatic impairment. [see Contraindications (4.5); Drug Interactions (7)]
Rhabdomyolysis
Simvastatin, lovastatin and atorvastatin are metabolized by CYP3A4. High levels of HMG-CoA reductase inhibitors increase the risk of myopathy and rhabdomyolysis. Avoid use of statins which are metabolized by CYP3A4 concomitantly with KETEK; suspend therapy with simvastatin, lovastatin, or atorvastatin during the course of treatment with KETEK. [see Drug Interactions (7)]
Hypotension
Hypotension, bradyarrhythmia and loss of consciousness have been observed in patients receiving concomitant treatment with calcium channel blockers that are substrates of CYP3A4 (e.g., verapamil, amlodipine, diltiazem). Monitor for these adverse reactions and toxicity related to calcium channel blockers and adjust calcium channel blocker dosage as necessary. [see Drug Interactions (7)]
5.5 Clostridum difficile-Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including KETEK, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
6 ADVERSE REACTIONS
The following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling:
- Myasthenia gravis [see Contraindications (4.1)]
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- QTc prolongation [see Warnings and Precautions (5.2)]
- Visual disturbances and loss of consciousness [see Warnings and Precautions (5.3)]
- Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Phase 3 clinical trials, 4,780 patients (n=2702 in controlled trials) received oral dosages of KETEK 800 mg once daily for 5 days or 7 to 10 days. Note that treatment with KETEK for 5 days duration is not a recommended dosage regimen. [see Dosage and Administration (2.1)]
In the combined Phase 3 studies, discontinuation due to adverse reactions occurred in 4.4% of KETEK-treated patients and 4.3% of combined comparator-treated patients. Most discontinuations in the KETEK group were due to adverse reactions in the gastrointestinal body system, primarily diarrhea (0.9% for KETEK vs. 0.7% for comparators), and nausea (0.7% for KETEK vs. 0.5% for comparators).
Adverse reactions (ARs) occurring in clinical studies in 2% or more of KETEK patients are included below.
| Adverse Reaction* | Percent Incidence | |
|---|---|---|
| KETEK n= 2702 | Comparator†
n= 2139 |
|
| Diarrhea | 10% | 8% |
| Nausea | 7% | 4.1% |
| Dizziness (excl. vertigo) | 2.8% | 1.5% |
| Vomiting | 2.4% | 1.4% |
Less Common Adverse Reactions
Frequency of 0.2% or more and less than 2%
The following adverse reactions were observed at a frequency of 0.2% or more and less than 2% in KETEK-treated patients in clinical studies.
- Gastrointestinal system: abdominal distension, dyspepsia, gastrointestinal upset, flatulence, constipation, gastroenteritis, gastritis, anorexia, oral candidiasis, glossitis, stomatitis.
- Liver and biliary system: abnormal liver function tests: increased transaminases (i.e., ALT, AST). Hepatitis, with or without jaundice, occurred in 0.07% of patients treated with KETEK. [see Warnings and Precautions (5.1)]
- Nervous system: dry mouth, somnolence, insomnia, vertigo, increased sweating
- Body as a whole: abdominal pain, fatigue
-
Special senses: Visual adverse reactions, some of them severe, most often included blurred vision, diplopia, or difficulty focusing. Some patients discontinued therapy due to these adverse reactions. Visual adverse reactions were reported as having occurred after any dose during treatment, but most (65%) occurred following the first or second dose. Visual adverse reactions lasted several hours and recurred upon subsequent dosing in some patients. For patients who continued treatment, some visual adverse reactions resolved on therapy while others persisted through the full course of treatment. [see Warnings and Precautions (5.3)]
Females and patients under 40 years old experienced a higher incidence of KETEK-associated visual adverse reactions. Table 2 provides the incidence of all visual adverse reactions in controlled Phase 3 studies by age and gender. The group with the highest incidence was females under the age of 40, while males over the age of 40 had rates of visual adverse reactions similar to comparator-treated patients.
| Gender/Age | Telithromycin | Comparators* |
|---|---|---|
|
||
| Female 40 and under | 2.1% (14/682) | 0.0% (0/534) |
| Female greater than 40 | 1.0% (7/703) | 0.35% (2/574) |
| Male 40 and under | 1.2% (7/563) | 0.48% (2/417) |
| Male greater than 40 | 0.27% (2/754) | 0.33% (2/614) |
| Total | 1.1% (30/2702) | 0.28% (6/2139) |
- Urogenital system: vaginal candidiasis, vaginitis, vaginosis fungal
- Skin: rash
- Hematologic: increased platelet count
Frequency of less than 0.2%
Other clinically-significant adverse reactions occurring in less than 0.2% of patients treated with KETEK from the controlled Phase 3 studies included: anxiety, bradycardia, eczema, elevated blood bilirubin, erythema multiforme, flushing, hypotension, increased blood alkaline phosphatase, increased eosinophil count, paresthesia, pruritus, urticaria.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of KETEK. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Allergic: face edema, severe allergic (hypersensitivity) reactions, including angioedema and anaphylaxis
- Cardiovascular: atrial arrhythmias, ventricular arrhythmias (including ventricular tachycardia and torsades de pointes) with potential fatal outcome, palpitation, ischemic cardiac events in the context of hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Gastrointestinal system: pseudomembranous colitis, pancreatitis [see Warnings and Precautions (5.5]
- Liver and biliary system: Hepatic dysfunction, fulminant hepatitis, hepatic necrosis, and hepatic failure, chromaturia [see Contraindications (4.2); Warnings and Precautions (5.1)]
- Musculoskeletal: muscle cramps, arthralgia, myalgia, exacerbation of myasthenia gravis [see Contraindications (4.1)]
- Nervous system: loss of consciousness, in some cases associated with vagal syndrome, tremor, convulsions
- Psychiatric disorders: confusion, hallucinations (mostly visual)
- Special senses: taste/smell perversion and/or loss, hearing loss
- Respiratory, thoracic and mediastinal disorders: dyspnea
7 DRUG INTERACTIONS
Telithromycin is a strong inhibitor of CYP3A4 and also a CYP3A4 substrate. Co-administration of KETEK and drugs that induce or inhibit the cytochrome P450 3A4 enzyme system may affect KETEK plasma concentrations resulting in diminished efficacy or an increase or prolongation of both the therapeutic and adverse effects; therefore, appropriate dosage adjustments may be necessary for drugs co-administered with telithromycin.
Studies were performed to evaluate the effect of CYP3A4 inhibitors on telithromycin and the effect of telithromycin on drugs that are substrates of CYP3A4 and CYP2D6. In addition, drug interaction studies were conducted with several other concomitantly prescribed drugs. Table 3 summarizes both drugs with pharmacokinetics that are affected by KETEK as well as drugs that affect the pharmacokinetics of KETEK.
| Drugs That Are Affected By KETEK | ||
| Drug(s) with Pharmacokinetics Affected by KETEK (Mechanism of interaction, if known) | Recommendation (Exposure) | Comments |
| Cisapride (CYP3A4 Substrate) | Contraindicated (Plasma exposure increased) | Co-administration of cisapride with repeated doses of KETEK resulted in significant increases in QTc. [see Contraindications (4.4)] |
| Pimozide (CYP3A4 Substrate) | Contraindicated (Plasma exposure likely to be increased) | Although there are no studies looking at the interaction between KETEK and pimozide, there is a potential risk of life-threatening QT prolongation. [see Contraindications (4.4)] |
| Colchicine
(CYP3A4 and P-glycoprotein efflux transporter Substrate) | Contraindicated in patients with renal or hepatic impairment. (Plasma exposure increased) | Risk of life-threatening colchicine toxicity. If co-administration of KETEK and colchicine is necessary in patients with normal renal and hepatic function, reduce the dose of colchicine. Monitor for symptoms of colchicine toxicity. [see Contraindications (4.5); Warnings and Precautions (5.5)] |
| Simvastatin, Lovastatin, and Atorvastatin (HMG-CoA Reductase Inhibitors metabolized by CYP3A4) (CYP3A4 Substrate) | Avoid Use (Plasma exposure increased) | High levels of HMG-CoA reductase inhibitors increase the risk of myopathy and rhabdomyolysis. Avoid concomitant use of simvastatin, lovastatin, or atorvastatin with KETEK. If KETEK is prescribed, suspend therapy with simvastatin, lovastatin, or atorvastatin during the course of KETEK. An interaction may occur with simvastatin, lovastatin or atorvastatin but not with statins which are not metabolized by CYP3A4. [see Warnings and Precautions (5.5)] |
| Ergot Alkaloids
| Not Recommended (Plasma exposure likely to be increased) | No specific drug interaction studies have been performed. However, acute ergot toxicity characterized by severe peripheral vasospasm and dysesthesia has been reported when macrolide antibiotics were co-administered with ergot alkaloid derivatives (such as ergotamine or dihydroergotamine). Without further data, the co-administration of KETEK and these drugs is not recommended. |
| Calcium Channel Blockers (CYP3A4 Substrate) | Use with Caution (Plasma exposure increased) | Hypotension, bradyarrhythmia, and loss of consciousness have been observed in patients receiving concomitant treatment with calcium channel blockers that are substrates of CYP3A4 (e.g., verapamil, amlodipine, diltiazem). Monitor for these adverse reactions and toxicity related to calcium channel blockers and adjust calcium channel blocker dosage as necessary. |
| Midazolam (CYP3A4 Substrate) | Use with Caution (Plasma exposure likely to be increased) | Monitor for benzodiazepine-related adverse reactions and adjust midazolam dosage if necessary. Use caution with other benzodiazepines, which are metabolized by CYP3A4 and undergo a high first-pass effect (e.g., triazolam). |
| Other drugs metabolized by CYP3A4, such as carbamazepine, cyclosporine, tacrolimus, sirolimus, hexobarbital, and phenytoin
(CYP3A4 Substrates) | Use with Caution (Plasma exposure likely to be increased) | No specific drug interaction studies have been performed to evaluate these drug-drug interactions with KETEK. However, increases or prolongation of the therapeutic and/or adverse effects of drugs metabolized by the cytochrome P450 system may be observed if administered with KETEK. |
| Metoprolol (CYP2D6 Substrate) | Use with Caution (Plasma exposure increased) | Co-administration of KETEK and metoprolol in patients with heart failure could lead to metoprolol toxicity and should be considered with caution. Monitor for metoprolol toxicity and adjust metoprolol dosage. |
| Digoxin | Use with Caution (Plasma exposure increased) | Monitor for digoxin side effects or serum levels during concomitant administration of digoxin and KETEK. |
| Theophylline
| Use with Caution (Plasma exposure minimally increased) | Co-administration of theophylline may worsen gastrointestinal effects such as nausea and vomiting, especially in female patients. Administer theophylline and KETEK 1 hour apart to decrease the likelihood of gastrointestinal side effects. |
| Oral Anticoagulants | Use with Caution (Plasma exposure increased) | Spontaneous post-marketing reports suggest that administration of KETEK and oral anticoagulants concomitantly may potentiate the effects of the oral anticoagulants. Consider monitoring prothrombin times/INR while patients are receiving KETEK and oral anticoagulants simultaneously. |
| Drugs that Affect KETEK | ||
| Drug(s) that Affect the Pharmacokinetics of KETEK | Recommendation | Comments |
| Rifampin (CYP3A4 Inducer) | Avoid Concomitant Use (Reduced KETEK exposure) | Loss of KETEK effect is likely [see Clinical Pharmacology (12.3)] |
| Other CYP3A4 inducers (phenytoin, carbamazepine, or phenobarbital) | Avoid Concomitant Use (Reduced KETEK exposure) | Loss of KETEK effect is likely [see Clinical Pharmacology (12.3)] |
| Itraconazole and Ketoconazole
| Avoid Concomitant Use (Increased KETEK exposure) | Increased KETEK toxicity is likely [see Clinical Pharmacology (12.3)] |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Telithromycin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Telithromycin was not teratogenic in the rat or rabbit. Reproduction studies have been performed in rats and rabbits, with effect on pre-post natal development studied in the rat. At doses of 150 and 20 mg/kg/day in rats and rabbits respectively (approximately 2 and 0.5 times the recommended clinical dose), no evidence of fetal terata was found. At doses higher than 150 or 20 mg/kg in rats and rabbits, respectively, maternal toxicity may have resulted in delayed fetal maturation. No adverse effects on prenatal and postnatal development of rat pups were observed at 125 mg/kg/day (1.5 times) the daily human dose.
8.3 Nursing Mothers
Telithromycin is excreted in breast milk of rats. Telithromycin may also be excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when KETEK is administered to a nursing mother.
8.4 Pediatric Use
The safety and effectiveness of KETEK in pediatric patients less than18 years of age has not been established. Pediatric clinical trials were halted prematurely due to concern of serious postmarketing hepatic adverse reactions observed in adults. [see Warnings and Precautions (5.1)]
8.5 Geriatric Use
Of the total number of patients in Phase 3 clinical trials (n=4,780), KETEK was administered to 694 patients who were 65 years and older, including 231 patients who were 75 years and older. Efficacy and safety in patients 65 years and older were generally similar to that observed in younger patients; however, greater sensitivity of some older individuals cannot be ruled out. [see Clinical Pharmacology (12.3)]
8.6 Renal and/or Hepatic Impairment
Dose adjustment is required in patients with severe renal impairment (CLCr less than 30 mL/min) or on dialysis. Further dose adjustment is required in patients with severe renal impairment and coexisting hepatic impairment. [see Dosage and Administration (2.2)]
10 OVERDOSAGE
In the event of acute overdosage, the stomach should be emptied by gastric lavage. The patient should be carefully monitored (e.g., ECG, electrolytes) and given symptomatic and supportive treatment. Adequate hydration should be maintained. The effectiveness of hemodialysis in an overdose situation with KETEK is unknown.
11 DESCRIPTION
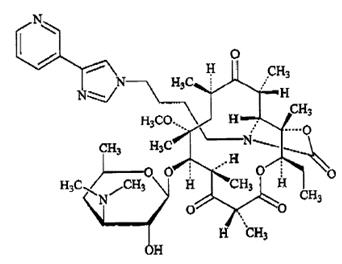
KETEK tablets contain telithromycin, a semisynthetic antibacterial in the ketolide class for oral administration. Chemically, telithromycin is designated as Erythromycin, 3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-11,12-dideoxy-6-O-methyl-3-oxo-12,11-[oxycarbonyl[[4-[4-(3-pyridinyl)-1H-imidazol-1-yl]butyl]imino]]-.
Telithromycin, a ketolide, differs chemically from the macrolide group of antibacterials by the lack of α-L-cladinose at position 3 of the erythronolide A ring, resulting in a 3-keto function. It is further characterized by a C11-12 carbamate substituted by an imidazolyl and pyridyl ring through a butyl chain. Its empirical formula is C43H65N5O10 and its molecular weight is 812.03. Telithromycin is a white to off-white crystalline powder. The following represents the chemical structure of telithromycin.
KETEK tablets are available as light-orange, oval, film-coated tablets, each containing 400 mg or 300 mg of telithromycin, and the following inactive ingredients: croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, red ferric oxide, talc, titanium dioxide, and yellow ferric oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Telithromycin is a ketolide antibacterial drug. [see Clinical Pharmacology (12.4)]
12.3 Pharmacokinetics
The pharmacokinetics of telithromycin after administration of single and multiple (7 days) once daily 800-mg doses to healthy adult subjects are shown in Table 4.
| Mean (SD) | ||
|---|---|---|
| Parameter | Single dose (n=18) | Multiple dose (n=18) |
| SD=Standard deviation ; Cmax=Maximum plasma concentration ; Tmax=Time to Cmax; AUC=Area under concentration vs. time curve; t1/2=Terminal plasma half-life; C24h =Plasma concentration at 24 hours post-dose | ||
|
||
| Cmax (µg/mL) | 1.9 (0.80) | 2.27 (0.71) |
| Tmax (h)* | 1.0 (0.5–4.0) | 1.0 (0.5–3.0) |
| AUC(0–24) (µg∙h/mL) | 8.25 (2.6) | 12.5 (5.4) |
| Terminal t1/2 (h) | 7.16 (1.3) | 9.81 (1.9) |
| C24h (µg/mL) | 0.03 (0.013) | 0.07 (0.051) |
In patients, mean peak and trough plasma concentrations were 2.9 µg/mL (±1.55), (n=219) and 0.2 µg/mL (±0.22), (n=204), respectively, after 3 to 5 days of KETEK 800 mg once daily. Steady-state plasma concentrations are reached within 2 to 3 days of once daily dosing with KETEK 800 mg.
Absorption
Following oral administration, telithromycin reached maximal concentration at about 1 hour (0.5 – 4 hours). KETEK has an absolute bioavailability of 57% in both young and elderly subjects.
The rate and extent of absorption are unaffected by food intake, thus KETEK tablets can be given without regard to food.
Distribution
Total in vitro protein binding is approximately 60% to 70% and is primarily due to human serum albumin.
Protein binding is not modified in elderly subjects or in patients with hepatic impairment.
The volume of distribution of telithromycin after intravenous infusion is 2.9 L/kg.
Telithromycin concentrations in bronchial mucosa, epithelial lining fluid, and alveolar macrophages after 800 mg once daily dosing for 5 days in patients are displayed in Table 5.
| Hours post-dose | Mean concentration (µg/mL) | Tissue/Plasma Ratio | ||
|---|---|---|---|---|
| Tissue or fluid | Plasma | |||
|
||||
| Bronchial mucosa | 2 | 3.88* | 1.86 | 2.11 |
| 12 | 1.41* | 0.23 | 6.33 | |
| 24 | 0.78* | 0.08 | 12.11 | |
| Epithelial lining fluid | 2 | 14.89 | 1.86 | 8.57 |
| 12 | 3.27 | 0.23 | 13.8 | |
| 24 | 0.84 | 0.08 | 14.41 | |
| Alveolar macrophages | 2 | 65 | 1.07 | 55 |
| 8 | 100 | 0.605 | 180 | |
| 24 | 41 | 0.073 | 540 | |
Metabolism
In total, approximately 70% of the telithromycin dose is metabolized. In plasma, the main circulating compound after administration of an 800-mg radio-labeled dose was parent compound, representing 56.7% of the total radioactivity. The main metabolite represented 12.6% of the AUC of telithromycin. Three other plasma metabolites were quantified, each representing 3% or less of the AUC of telithromycin.
It is estimated that approximately 50% of its metabolism is mediated by CYP3A4 and the remaining 50% is CYP -independent.
Excretion
The systemically available telithromycin is eliminated by multiple pathways as follows: 7% of the dose is excreted unchanged in feces by biliary and/or intestinal secretion; 13% of the dose is excreted unchanged in urine by renal excretion; and 37% of the dose is metabolized by the liver. Following oral dosing, the mean terminal elimination half-life of telithromycin is 10 hours.
Specific Populations
Gender: There was no significant difference between males and females in mean AUC, Cmax, and elimination half-life in two studies; one in 18 healthy young volunteers (18 to 40 years of age) and the other in 14 healthy elderly volunteers (65 to 92 years of age), given single and multiple once daily doses of 800 mg of KETEK.
Hepatic impairment: Telithromycin is excreted via the liver and kidney. [see Dosage and Administration (2.2)]
In a single-dose study (800 mg) in 12 patients and a multiple-dose study (800 mg) in 13 patients with mild to severe hepatic insufficiency (Child Pugh Class A, B and C), the Cmax, AUC and t1/2 of telithromycin were similar to those obtained in age- and sex-matched healthy subjects. In both studies, an increase in renal elimination was observed in hepatically impaired patients indicating that this pathway may compensate for some of the decrease in metabolic clearance.
Renal impairment: Telithromycin is excreted via the liver and kidney. [see Dosage and Administration (2.2)]
In a multiple-dose study, 36 subjects with varying degrees of renal impairment received 400 mg, 600 mg, or 800 mg KETEK once daily for 5 days. There was a 1.4-fold increase in Cmax,ss, and a 1.9-fold increase in AUC (0–24)ss at 800 mg multiple doses in the severely renally impaired group (CLCR less than 30 mL/min) compared to healthy volunteers. Renal excretion may serve as a compensatory elimination pathway for telithromycin in situations where metabolic clearance is impaired. Patients with severe renal impairment are prone to conditions that may impair their metabolic clearance.
In a single-dose study in patients with end-stage renal failure on hemodialysis (n=10), the mean Cmax and AUC values were similar to normal healthy subjects when KETEK was administered 2 hours post-dialysis. However, the effect of dialysis on removing telithromycin from the body has not been studied.
Combined Renal and Hepatic Impairment: The effects of co-administration of ketoconazole in 12 subjects (age 60 years and older), with impaired renal function were studied (CLCR= 24 to 80 mL/min). In this study, when severe renal insufficiency (CLCR less than 30 mL/min, n=2) and concomitant impairment of CYP3A4 metabolism pathway were present, telithromycin exposure (AUC0–24) was increased by approximately 4- to 5-fold compared with the exposure in healthy subjects with normal renal function receiving telithromycin alone. In the presence of severe renal impairment (CLCR less than 30 mL/min), with coexisting hepatic impairment, a reduced dosage of KETEK is recommended. [see Dosage and Administration (2.2)]
Geriatric: Pharmacokinetic data show that there is an increase of 1.4-fold in exposure (AUC) in 20 patients 65 years and older with community acquired pneumonia in a Phase 3 study, and a 2.0-fold increase in exposure (AUC) in 14 subjects 65 years and older as compared with subjects less than 65 years of age in a Phase I study. No dosage adjustment is required based on age alone. [see Use in Specific Populations (8.5)]
Drug Interactions
CYP3A4 inducers
Rifampin
During concomitant administration of rifampin and KETEK in repeated doses, Cmax and AUC of telithromycin were decreased by 79%, and 86%, respectively. [see Drug Interactions (7)]
CYP3A4 inhibitors
Itraconazole: A multiple-dose interaction study with itraconazole showed that Cmax of telithromycin was increased by 22% and AUC by 54%. [see Drug Interactions (7)]
Ketoconazole: A multiple-dose interaction study with ketoconazole showed that Cmax of telithromycin was increased by 51% and AUC by 95%. [see Drug Interactions (7)]
CYP3A4 substrates
Simvastatin: When simvastatin was co-administered with telithromycin, there was a 5.3-fold increase in simvastatin Cmax, an 8.9-fold increase in simvastatin AUC, a 15-fold increase in the simvastatin active metabolite Cmax, and a 12-fold increase in the simvastatin active metabolite AUC. In another study, when simvastatin and telithromycin were administered 12 hours apart, there was a 3.4-fold increase in simvastatin Cmax, a 4.0-fold increase in simvastatin AUC, a 3.2-fold increase in the active metabolite Cmax, and a 4.3-fold increase in the active metabolite AUC. [see Warnings and Precautions (5.4); Drug Interactions (7)]
Midazolam: Concomitant administration of telithromycin with intravenous or oral midazolam resulted in 2- and 6-fold increases, respectively, in the AUC of midazolam due to inhibition of CYP3A4-dependent metabolism of midazolam. [see Drug Interactions (7)]
Other Drugs
Digoxin
The plasma peak and trough levels of digoxin were increased by 73% and 21%, respectively, in healthy volunteers when co-administered with KETEK. However, trough plasma concentrations of digoxin (when equilibrium between plasma and tissue concentrations has been achieved) ranged from 0.74 to 2.17 ng/mL. There were no significant changes in ECG parameters and no signs of digoxin toxicity. [see Drug Interactions (7)]
Theophylline
When theophylline was co-administered with repeated doses of KETEK, there was an increase of approximately 16% and 17% on the steady-state Cmax and AUC of theophylline. [see Drug Interactions (7)]
Sotalol
KETEK has been shown to decrease the Cmax and AUC of sotalol by 34% and 20%, respectively, due to decreased absorption.
Oral Contraceptives
When oral contraceptives containing ethinyl estradiol and levonorgestrel were co-administered with KETEK, the steady-state AUC of ethinyl estradiol did not change and the steady-state AUC of levonorgestrel was increased by 50%. The pharmacokinetic/pharmacodynamic study showed that telithromycin did not interfere with the antiovulatory effect of oral contraceptives containing ethinyl estradiol and levonorgestrel.
Metoprolol
When metoprolol was co-administered with KETEK, there was an increase of approximately 38% on the Cmax and AUC of metoprolol; however, there was no effect on the elimination half-life of metoprolol. Telithromycin exposure is not modified with concomitant single-dose administration of metoprolol. [see Drug Interactions (7)]
Ranitidine/Antacid
There was no clinically relevant pharmacokinetic interaction of ranitidine or antacids containing aluminum and magnesium hydroxide on telithromycin.
Cisapride
Steady state peak plasma concentrations of cisapride (an agent with the potential to increase QT interval) were increased by 95% when co-administered with repeated doses of telithromycin, resulting in significant increases in QTc. [see Contraindications (4.4)]
OATP1B1 and OATP1B3
In vitro studies using a model compound have shown that telithromycin may act as an inhibitor for the hepatic uptake transporters OATP1B1 and OATP1B3. Although the clinical relevance of this finding is unknown, it is possible that concomitant administration of KETEK with drugs that are substrates of OATP family members could result in increased plasma concentrations of the co-administered drug.
12.4 Microbiology
Mechanism of Action
Telithromycin belongs to the ketolide class of antibacterials and is structurally related to the macrolides. Telithromycin blocks protein synthesis by binding to domains II and V of 23S rRNA of the 50S ribosomal subunit. Telithromycin may also inhibit the assembly of nascent ribosomal units.
Telithromycin concentrates in phagocytes where it exhibits activity against intracellular respiratory pathogens. In vitro, telithromycin has been shown to demonstrate concentration-dependent bactericidal activity against isolates of Streptococcus pneumoniae (including multi-drug resistant isolates [MDRSP2]).
- 2
- MDRSP=Multi-drug resistant Streptococcus pneumoniae includes isolates known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are isolates resistant to two or more of the following antimicrobials: penicillin, 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines, and trimethoprim/sulfamethoxazole.
Mechanism of Resistance
Production of Erm dimethyltransferases may cause telithromycin resistance in some Gram-positive bacteria.
List of Microorganisms
Telithromycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical settings [see Indications and Usage (1)].
-
Gram-positive bacteria
Streptococcus pneumoniae (including MDRSP)
-
Gram-negative bacteria
Haemophilus influenzae
Moraxella catarrhalis
-
Other microorganisms
Chlamydophila pneumoniae
Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit in vitro minimum inhibitory concentrations (MICs) less than or equal to the susceptible breakpoint for telithromycin. However, the safety and efficacy of KETEK in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
-
Gram-positive bacteria
Staphylococcus aureus (methicillin and erythromycin susceptible isolates only)
Streptococcus pyogenes (erythromycin susceptible isolates only)
Beta-hemolytic streptococci (Lancefield groups C and G)
-
Other microorganisms
Legionella pneumophila
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide cumulative results of in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antibacterial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on dilution methods (broth or agar dilution)1,3 or equivalent with standardized inoculum and concentrations of telithromycin powder. The MIC values should be interpreted according to criteria provided in Table 6.
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antibacterials. One such standardized procedure2,3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15 µg telithromycin to test the susceptibility of microorganisms to telithromycin. Disc diffusion zone sizes should be interpreted according to criteria in Table 6.
| Pathogen | Minimum Inhibitory Concentrations (µg/mL) | Disk Diffusion Zone Diameter (mm) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| Streptococcus pneumoniae | ≤1 | 2 | ≥4 | ≥19 | 16–18 | ≤15 |
| Haemophilus influenzae | ≤4 | 8 | ≥16 | ≥15 | 12–14 | ≤11 |
A report of "Susceptible" indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antibacterial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Quality control
Standardized susceptibility test procedures require the use of quality control microorganisms to determine the performance of the test procedures1,2,3. Standard telithromycin powder should provide the MIC ranges for the quality control organisms in Table 7. For the disk diffusion technique, the 15-μg telithromycin disk should provide the zone diameter ranges for the quality control organisms in Table 7.
| Quality Control Organism | Minimum Inhibitory Concentrations (µg/mL) | Disk Diffusion (zone diameter in mm) |
|---|---|---|
| ATCC = American Type Culture Collection | ||
| Haemophilus influenzae
ATCC 49247 | 1.0–4.0 | 17–23 |
| Streptococcus pneumoniae
ATCC 49619 | 0.004–0.03 | 27–33 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to determine the carcinogenic potential of KETEK have not been conducted.
Telithromycin showed no evidence of genotoxicity in four tests: gene mutation in bacterial cells, gene mutation in mammalian cells, chromosome aberration in human lymphocytes, and the micronucleus test in the mouse.
No evidence of impaired fertility in the rat was observed at doses estimated to be 0.6 times the human daily dose on a body surface area basis (50 mg/kg/day). At doses of 2–4 times the human daily dose (150 and 300 mg/kg/day, at which signs of parental toxicity were observed), moderate reductions in fertility indices were noted in male and female animals treated with telithromycin.
13.2 Animal Toxicology and/or Pharmacology
Repeated dose toxicity studies of 1, 3, and 6 months' duration with telithromycin conducted in rat, dog and monkey showed that the liver was the principal target for toxicity with elevations of liver enzymes and histological evidence of damage. There was evidence of reversibility after cessation of treatment. Plasma exposures based on free fraction of drug at the no observed adverse effect levels ranged from 1 to 10 times the expected clinical exposure.
Phospholipidosis (intracellular phospholipid accumulation) affecting a number of organs and tissues (e.g., liver, kidney, lung, thymus, spleen, gall bladder, mesenteric lymph nodes, GI-tract) has been observed with the administration of telithromycin in rats at repeated doses of 150 mg/kg/day ( 2× the human dose on a body surface area basis) or more for 1 month, and 50 mg/kg/day (0.6× the human dose) or more for 3–6 months. Similarly, phospholipidosis has been observed in dogs with telithromycin at repeated doses of 150 mg/kg/day (6× the human dose on a body surface area basis) or more for 1 month and 50 mg/kg/day (2× the human dose) or more for 3 months. The significance of these findings for humans is unknown.
Pharmacology/toxicology studies showed an effect both in prolonging QTc interval in dogs in vivo and in vitro action potential duration (APD) in rabbit Purkinje fibers. These effects were observed at concentrations of free drug at least 8.8 (in dogs) times those circulating in clinical use. In vitro electrophysiological studies (hERG assays) suggested an inhibition of the rapid activating component of the delayed rectifier potassium current (IKr) as an underlying mechanism.
14 CLINICAL STUDIES
KETEK was studied in four randomized, double-blind, controlled studies and four open-label studies for the treatment of community-acquired pneumonia (CAP). Patients with mild to moderate CAP who were considered appropriate for oral outpatient treatment were enrolled in these trials. Patients with severe pneumonia were excluded based on any one of the following: ICU admission, need for parenteral antibacterials, respiratory rate greater than 30 per minute, hypotension, altered mental status, less than 90% oxygen saturation by pulse oximetry, or white blood cell count less than 4000 per mm3. There were 2016 clinically evaluable patients in the KETEK group.
| Patients (n) | Clinical Cure Rate | |||
|---|---|---|---|---|
| Controlled Studies | KETEK | Comparator | KETEK | Comparator |
|
||||
| KETEK vs. clarithromycin 500 mg twice a day for 10 days | 162 | 156 | 88.3% | 88.5% |
| KETEK vs. trovafloxacin* 200 mg daily for 7 to 10 days | 80 | 86 | 90.0% | 94.2% |
| KETEK vs. amoxicillin 1000 mg three times a day for 10 days | 149 | 152 | 94.6% | 90.1% |
| KETEK for 7 days vs. clarithromycin 500 mg twice a day for 10 days | 161 | 146 | 88.8% | 91.8% |
Clinical cure rates by pathogen from the four CAP controlled clinical trials in microbiologically evaluable patients given KETEK for 7–10 days or a comparator are displayed in Table 9.
| Pathogen | KETEK | Comparator |
|---|---|---|
| Streptococcus pneumoniae | 73/78 (93.6%) | 63/70 (90%) |
| Haemophilus influenzae | 39/47 (83%) | 42/44 (95.5%) |
| Moraxella catarrhalis | 12/14 (85.7%) | 7/9 (77.8%) |
| Chlamydophila pneumoniae | 23/25 (92%) | 18/19 (94.7%) |
| Mycoplasma pneumoniae | 22/23 (95.7%) | 20/22 (90.9%) |
Clinical cure rates for patients with CAP due to Streptococcus pneumoniae were determined from patients in controlled and uncontrolled trials. Of 333 evaluable patients with CAP due to Streptococcus pneumoniae, 312 (93.7%) achieved clinical success. Blood cultures were obtained in all patients participating in the clinical trials of mild to moderate community-acquired pneumonia. In a limited number of outpatients with incidental pneumococcal bacteremia treated with KETEK, a clinical cure rate of 88% (67/76) was observed. KETEK is not indicated for the treatment of severe community-acquired pneumonia or suspected pneumococcal bacteremia.
Clinical cure rates for patients with CAP due to multi-drug resistant Streptococcus pneumoniae (MDRSP3) were determined from patients in controlled and uncontrolled trials. Of 36 evaluable patients with CAP due to MDRSP, 33 (91.7%) achieved clinical success.
| Screening Susceptibility | Clinical Success in Evaluable MDRSP Patients | |
|---|---|---|
| n/N* | % | |
| Penicillin-resistant | 20/23 | 86.9 |
| 2nd generation cephalosporin-resistant | 20/22 | 90.9 |
| Macrolide-resistant | 25/28 | 89.3 |
| Trimethoprim/ sulfamethoxazole-resistant | 24/27 | 88.9 |
| Tetracycline-resistant† | 11/13 | 84.6 |
- 3
- MDRSP: Multi-drug resistant Streptococcus pneumoniae includes isolates known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are isolates resistant to two or more of the following antibacterials: penicillin, 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
15 References
- 1.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Ninth Edition. CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- 2.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Eleventh Edition. CLSI document M02-A11, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- 3.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement, CLSI document M100-S24, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2014.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
KETEK® 400 mg tablets are supplied as light-orange, oval, film-coated tablets, imprinted "H3647" on one side and "400" on the other side.
| Bottles of 60 | (NDC 0088-2225-41) |
KETEK® 300 mg tablets are supplied as light-orange, oval, film-coated tablets, imprinted "38AV" on one side and blank on the other side.
| Bottles of 20 | (NDC 0088-2223-20) |
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (MEDICATION GUIDE).
Communicate the following information and instructions to the patient:
- Drug Resistance
Antibacterial drugs including KETEK should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When KETEK is prescribed to treat a bacterial infection, inform patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by KETEK or other antibacterial drugs in the future.
- Myasthenia Gravis
Advise patients not to take KETEK if they have myasthenia gravis [see Contraindications (4.1)]
- Liver Injury
Advise patients of the possibility of severe liver injury, associated with KETEK. Instruct them to discontinue KETEK and seek medical attention immediately if they develop nausea, fatigue, anorexia, jaundice, dark urine, light-colored stools, pruritus, or tender abdomen. These problems may occur after any dose during treatment or after treatment had stopped. Advise patients not to take KETEK if they have a previous history of hepatitis/jaundice associated with the use of KETEK or macrolide antibacterials. [see Contraindications (4.2); Warnings and Precautions (5.1)]
- Changes in Electrocardiogram
KETEK may produce changes in the electrocardiogram (QTc interval prolongation). Advise patient to report any fainting or palpitations occurring during drug treatment. [see Warnings and Precautions (5.2); Adverse Reactions (6.2)]
Advise patients to avoid KETEK if they are receiving Class 1A (e.g., quinidine, procainamide) or Class III (e.g., dofetilide) antiarrhythmic agents.
- Problems with Vision and Loss of Consciousness
KETEK may cause blurred vision, difficulty focusing, and objects looking doubled. These problems may occur after any dose during treatment, last for several hours, and come back with the next dose. [See Warnings and Precautions (5.3); Adverse Reactions (6.1)]
KETEK may also cause transient loss of consciousness. [See Warnings and Precautions (5.3)]
Advise patients to avoid quick changes in viewing between objects in the distance and objects nearby to help decrease the effects of these visual difficulties.
Advise patients to minimize activities such as driving a motor vehicle, operating heavy machinery or engaging in other hazardous activities during treatment with KETEK, because of potential visual difficulties, loss of consciousness, confusion or hallucinations.
Advise patients that if visual difficulties, loss of consciousness / fainting, confusion or hallucination occur, to seek advice from their physician before taking another dose and to refrain from hazardous activities.
- Drug/Food Interactions
Advise patients that KETEK tablets can be taken with or without food.
Colchicine should be avoided in patients receiving KETEK. Advise patients with normal kidney and liver function that the dose of colchicine should be reduced while they are taking KETEK. [see Warnings and Precautions (5.4); Drug Interactions (7)]
Simvastatin, lovastatin, or atorvastatin should be avoided in patients receiving KETEK. Advise patients that KETEK therapy with simvastatin, lovastatin, or atorvastatin should be stopped during the course of treatment with KETEK due to increased risk of rhabdomyolysis. [see Warnings and Precautions (5.4); Drug Interactions (7)]
Taking KETEK with calcium channel blockers may cause severe hypotension, bradycardia and loss of consciousness. Advise patients that if these symptoms occur to contact their physician as soon as possible. [see Warnings and Precautions (5.4); Drug Interactions (7)]
Advise patients to inform their physician of any other medications taken concurrently with KETEK, including over-the-counter medications and dietary supplements.
- Diarrhea
Diarrhea is a common problem caused by antibacterials including KETEK which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible. [see Warnings and Precautions (5.5)]
Manufactured for:
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
A SANOFI COMPANY
© 2015 sanofi-aventis U.S. LLC
MEDICATION GUIDE
KETEK® (KEE tek)
(telithromycin)
Tablets
Read this Medication Guide before you start taking KETEK and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT KETEK?
KETEK can cause serious side effects, including:
- Worsening of myasthenia gravis symptoms in people who already have myasthenia gravis (a disease which causes muscle weakness). Worsening of myasthenia gravis symptoms, including life-threatening breathing problems, have happened in people with myasthenia gravis after taking KETEK. Some life-threatening breathing problems have caused death. Do not take KETEK if you have myasthenia gravis.
WHAT IS KETEK?
KETEK is a prescription medication used to treat mild to moderate community-acquired pneumonia in adults 18 years of age and older.
- KETEK is only used to treat certain types of bacteria and is not meant for use to treat all types of bacterial infections.
It is not known if KETEK is safe and effective in children.
Who should not take KETEK?
Do not take KETEK if you:
- have myasthenia gravis.
- have had liver problems or yellowing of your eyes and/or skin (jaundice) while taking KETEK or macrolide antibacterials.
- are allergic to KETEK, or macrolide antibacterials.
- take cisapride or pimozide.
- take colchicine and have kidney or liver problems.
What should I tell my doctor before taking KETEK?
Before you take KETEK, tell your doctor if you:
- have or have had liver or kidney problems
- have a heart problem called "QTc prolongation" or have a family history of QTc prolongation
- have other heart problems
- are pregnant or plan to become pregnant. It is not known if KETEK will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. It is not known if KETEK passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take KETEK.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking KETEK with other medicines can cause serious side effects. Ask your doctor for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take KETEK?
- Take KETEK exactly as your doctor tells you to take it.
- If you have kidney problems, your doctor may prescribe a lower dose of KETEK for you.
- Take KETEK with or without food.
- If you take too much KETEK, call your doctor, or go to the nearest hospital emergency room right away.
What should I avoid while taking KETEK?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how KETEK affects you.
What are the possible side effects of KETEK?
KETEK may cause serious side effects, including:
- See "What is the most important information I should know about KETEK?"
-
severe liver problems and severe liver damage (hepatotoxicity) that can lead to liver transplant or death. Severe liver problems can happen while you take KETEK, even after a few doses or right after you stop taking it. Symptoms of liver problems may include:
- loss of appetite
- increased tiredness
- nausea
- yellowing of your skin or white of your eyes
- dark colored urine (tea colored)
- light colored stools
- right upper belly (abdomen) pain
- a heart problem called QTc prolongation that can lead to death. Symptoms of QTc prolongation include fainting and fast heartbeat (heart palpitations). Call your doctor right away if you have these symptoms.
- vision problems. KETEK may cause you to have blurred vision, trouble focusing your eyes, and double vision. You may especially notice vision problems if you look quickly between objects close to you and objects far away from you.
- fainting. KETEK may cause you to faint, especially if you also have nausea, vomiting, and lightheadedness (vagal syndrome). See "What should I avoid while taking KETEK?"
- drug interaction with colchicine in people with normal kidney and liver function that may lead to death.
-
severe muscle damage (rhabdomyolysis). KETEK may cause rhabdomyolysis when you also take certain medicines used to treat high levels of cholesterol in your blood. These medicines include:
- simvastatin
- lovastatin
- atorvastatin
-
low blood pressure, slow heart rate, and fainting. KETEK may cause you to have low blood pressure, a slow heart rate, and fainting when you also take certain medicines called calcium channel blockers. Calcium channel blockers include:
- verapamil
- amlodipine
- diltiazem
- or other medicines containing these products
-
an intestinal infection (Clostridium difficile-associated diarrhea). Clostridium difficile-associated diarrhea can happen up to 2 months after you have stopped taking KETEK. Symptoms of Clostridium difficile-associated diarrhea may include:
- watery diarrhea
- diarrhea that does not go away
- bloody stools
- stomach cramps
- fever
Stop taking KETEK and call your doctor right away if you have any of these symptoms listed above. Do not take another dose of KETEK unless your doctor tells you to.
The most common side effects of KETEK include:
- diarrhea
- nausea
- dizziness
- vomiting
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of KETEK. For more information ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store KETEK?
- Store KETEK tablets at room temperature, between 59°F to 86°F (15°C to 30°C).
- Keep KETEK and all medicines out of the reach of children.
General information about the safe and effective use of KETEK
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use KETEK for a condition for which it was not prescribed. Do not share KETEK with other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about KETEK. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about KETEK that is written for health professionals. For more information, go to www.KETEK.com or call 1-800-446-6267.
What are the ingredients in KETEK?
Active Ingredient: telithromycin
Inactive Ingredients: croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, red ferric oxide, talc, titanium dioxide, and yellow ferric oxide
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for:
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
A SANOFI COMPANY
Revised: December 2015
© 2015 sanofi-aventis U.S. LLC
| KETEK
telithromycin tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| KETEK
telithromycin tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (783243835) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanofi-aventis U.S. LLC | 783243835 | MANUFACTURE(0088-2225, 0088-2223) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AAI Pharma Services Corp. | 832395235 | MANUFACTURE(0088-2225, 0088-2223) , ANALYSIS(0088-2225, 0088-2223) , LABEL(0088-2225, 0088-2223) , PACK(0088-2225, 0088-2223) | |

