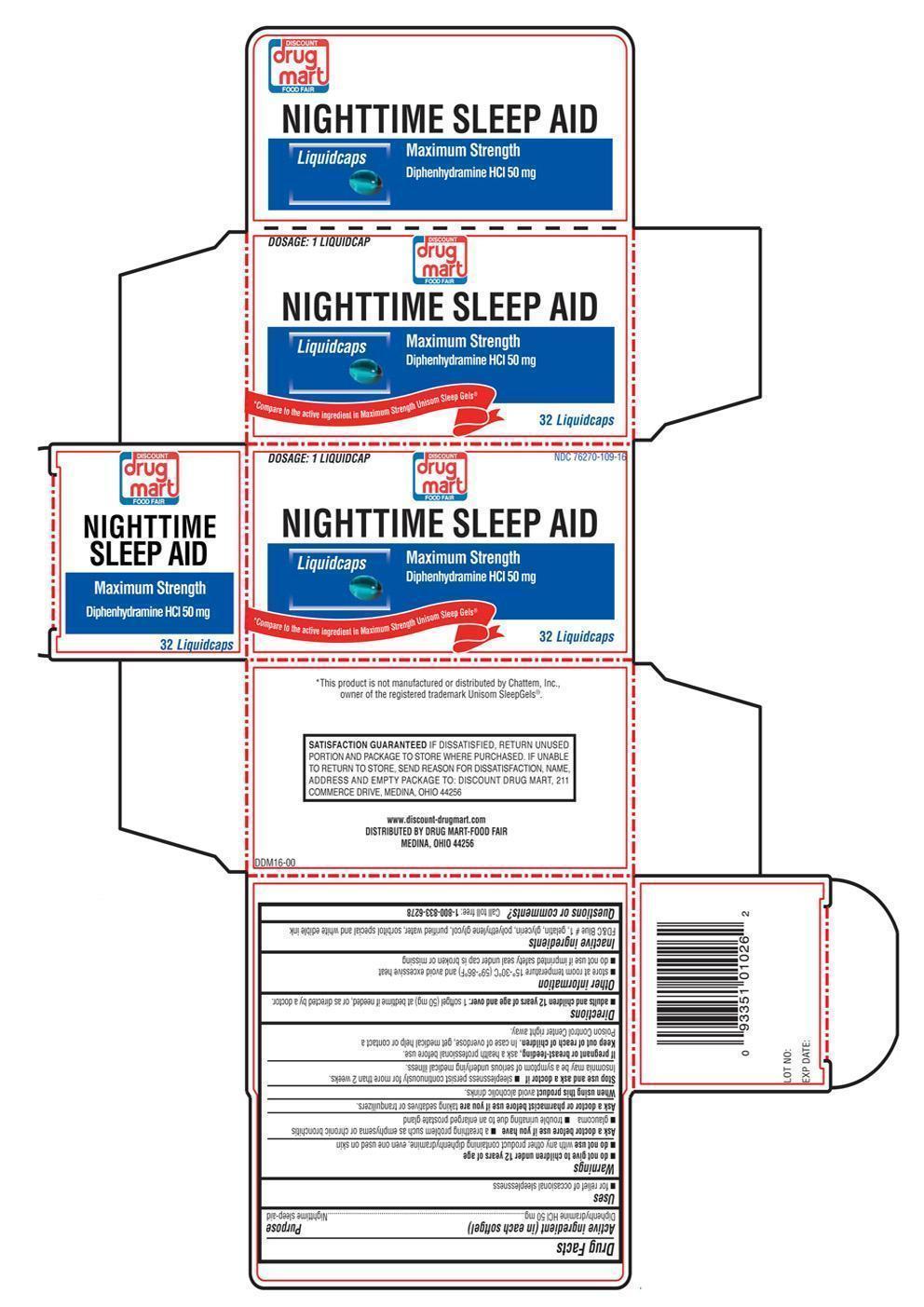
NIGHTTIME SLEEP AID- diphenhydramine hydrochloride 50 mg capsule, liquid filled
PuraVation Pharmaceuticals Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NIGHTTIME SLEEP AID capsule, liquid filled
Warnings
- do not give to children under 12 years of age
- do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- adults and children 12 years of age and over: 1 softgel (50 mg) at bedtime if needed, or as directed by a doctor.
Other information
- store at room temperature 15°-30°C (59°-86°F) and avoid excessive heat
- do not use if imprinted safety seal under cap is broken or missing
| NIGHTTIME SLEEP AID
diphenhydramine hydrochloride 50 mg capsule, liquid filled |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PuraVation Pharmaceuticals Inc (968385034) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humanwell PuraCap Pharmaceutical (Wuhan), Ltd. | 421293287 | MANUFACTURE(76270-109) , ANALYSIS(76270-109) | |
Revised: 12/2016
Document Id: 6080b6c6-0dd1-4c57-b62f-cafc881b9b2a
Set id: 4400053d-e06f-44f0-9842-835438e2461a
Version: 2
Effective Time: 20161230
PuraVation Pharmaceuticals Inc