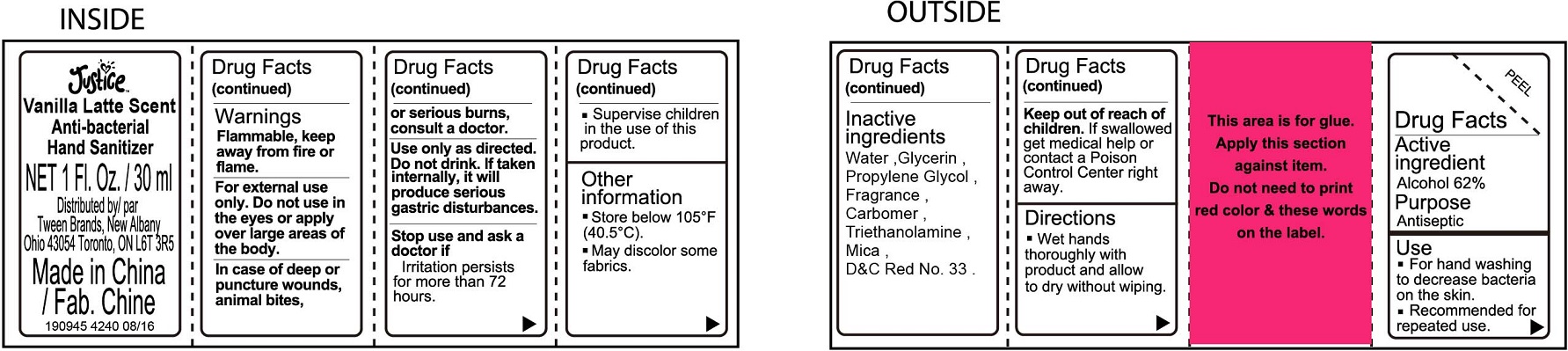
JUSTICE VANILLA LATTE SCENTED ANTI-BACTERIAL HAND SANITIZER- alcohol gel
Tween Brands, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Justice Vanilla Latte Scented Anti-Bacterial Hand Sanitizer
Warnings
Flammable, keep away from fire or flame.
For external use only.
Directions
- Wet hands thoroughly with product and allow to dry without wiping.
- Supervise children in the use of this product.
| JUSTICE VANILLA LATTE SCENTED ANTI-BACTERIAL HAND SANITIZER
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Tween Brands, Inc (965758188) |
Revised: 11/2022
Document Id: ed3ff4ec-7c66-c4c5-e053-2995a90ad9d7
Set id: 40e36b07-b7f8-4e09-a36d-46c9fd8d1082
Version: 5
Effective Time: 20221112
Tween Brands, Inc