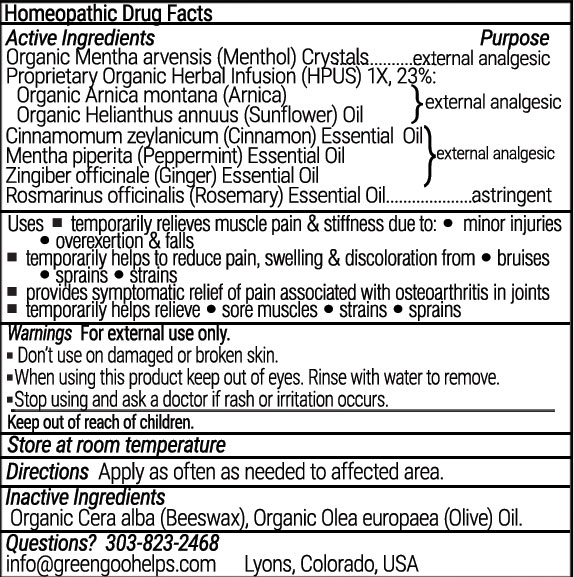
PAIN RELIEF- arnica, menthol crystals, cinnamon, peppermint, ginger, rosemary salve
SnugsZ USA
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Pain Relief Homeopathic Salve
| PAIN RELIEF
arnica, menthol crystals, cinnamon, peppermint, ginger, rosemary salve |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SnugsZ USA (615959228) |
| Registrant - Sierra Sage Herbs LLC (006970452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SnugsZ USA | 615959228 | manufacture(76309-102) , label(76309-102) , pack(76309-102) | |
Revised: 2/2017
Document Id: 480dc24e-87c2-55db-e054-00144ff88e88
Set id: 40800562-68ef-45be-e054-00144ff88e88
Version: 14
Effective Time: 20170208
SnugsZ USA