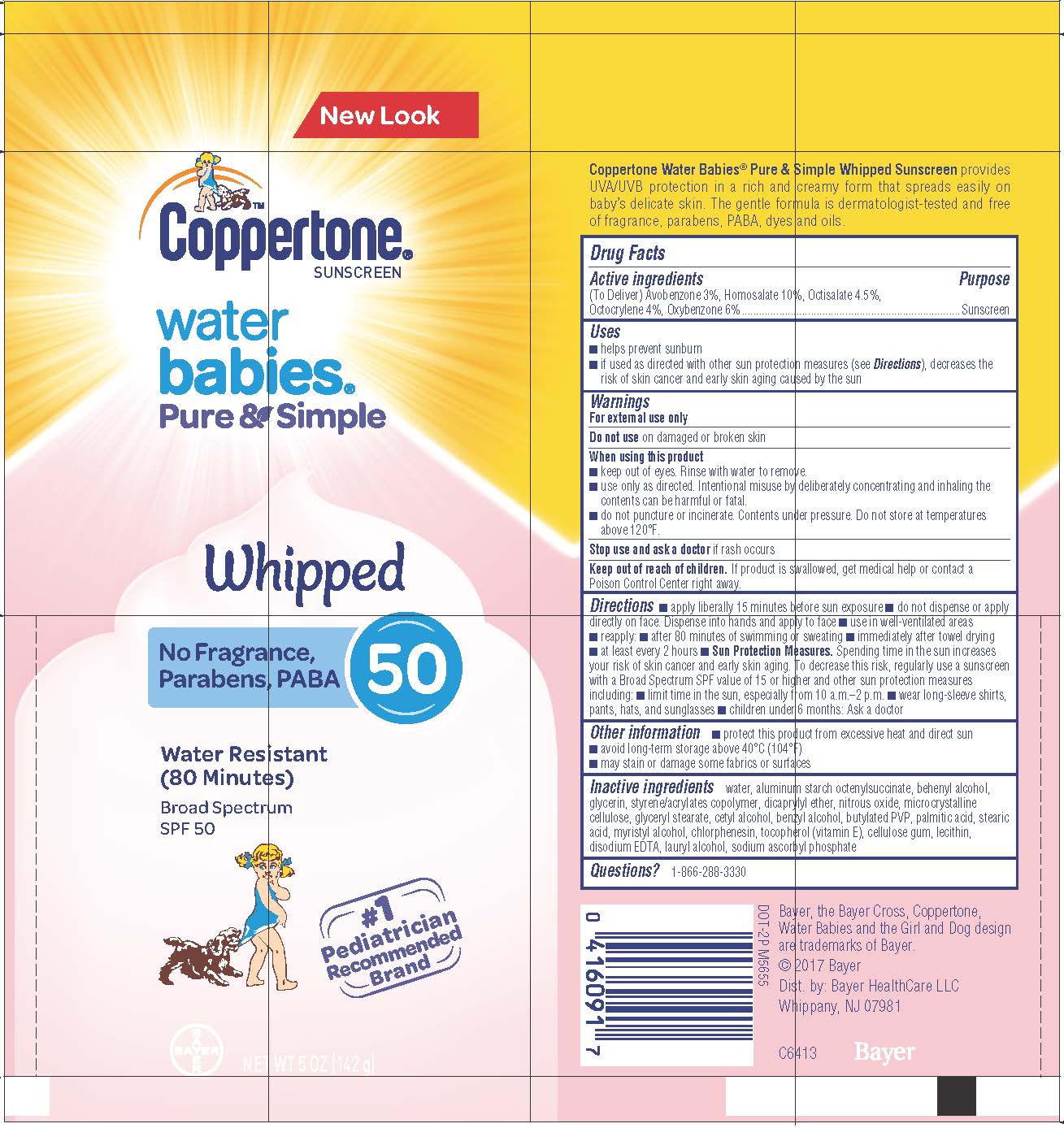
COPPERTONE WATER BABIES PURE AND SIMPLE WHIPPED SPF 50- avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion
Bayer Healthcare LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Coppertone Water Babies Pure & Simple Whipped SPF 50
Active Ingredients
Active ingredients
(To Deliver) Avobenzone 3%, Homosalate 10%, Octisalate 4.5%,
Octocrylene 4%, Oxybenzone 6%
Uses
Uses
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases
the risk of skin cancer and early skin aging caused by the sun
When using this product
When using this product
- keep out of eyes. Rinse with water to remove.
- use only as directed. Intentional misuse by deliberately concentrating and inhaling
the contents can be harmful or fatal.
- do not puncture or incinerate. Contents under pressure. Do not store at
temperatures above 120°F.
Keep out of reach of children
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions
apply liberally 15 minutes before sun exposure
do not dispense or apply directly on face. Dispense into hands and apply to face
use in well-ventilated areas
reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 and higher and other sun protection measures including:
- limit time in the sun, expecially from 10 a.m.-2 p.m.
- wear long-sleeve shirts, pant, hats, and sunglasses
children under 6 months: Ask a doctor
Other information
Other information
- protect this product from excessive heat and direct sun
- avoid long term storage above 40°C (104°F)
- may stain or damage some fabrics or surfaces
Inactive Ingredients
Inactive Ingredients Water, Aluminum Starch Octenylsuccinate, Behenyl Alcohol, Glycerin, Styrene/Acrylates Copolymer, Dicaprylyl Ether, Microcrystalline Cellulose, Glyceryl Stearate, Cetyl Alcohol, Benzyl Alcohol, Butylated PVP, Palmitic Acid, Stearic Acid, Myristyl Alcohol, Chlorphenesin, Tocopherol (Vitamin E), Cellulose Gum, Lecithin, Disodium EDTA, Lauryl Alcohol, Sodium Ascorbyl Phosphate, Nitrous Oxide.
| COPPERTONE WATER BABIES PURE AND SIMPLE WHIPPED
SPF 50
avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Bayer Healthcare LLC. (112117283) |