CLEARCALM 3 ANTI-ACNE STARTER- sulfur cream
Ren Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ClearCalm 3 Anti-Acne Starter Kit
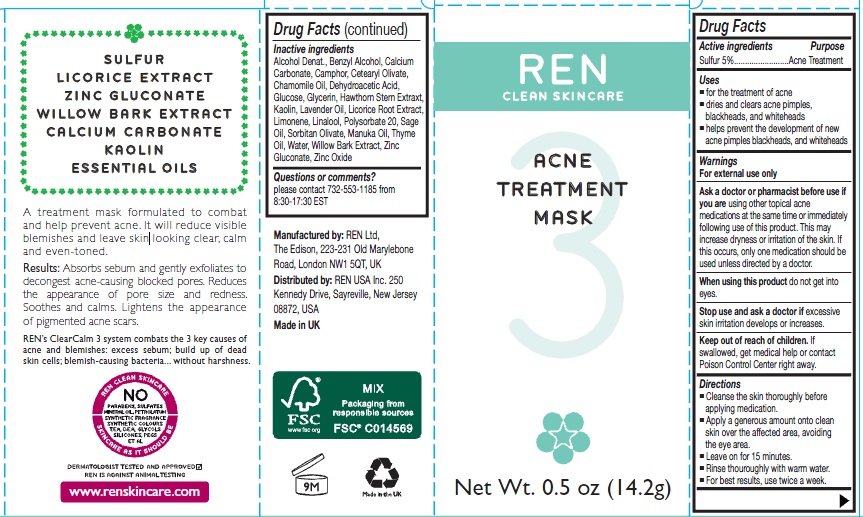
Uses
- for treatment of acne
- dries and clears acne pimples, blackheads, and whiteheads
- helps prevent development of new acne pimples, blackheads, and whiteheads
Ask a doctor or pharmacist before use if you are
using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
- Cleanse the skin thoroughly before applying medication.
- Apply a generous amount onto clean skin over the affected area, avoiding the eye area.
- Leave on for 15 minutes.
- Rinse thoroughly with warm water.
- For best results, use twice a week.
Inactive Ingredients
Alcohol Denat, Benzyl Alcohol, Calcium Carbonate, Camphor, CetearylOlivate, Chamomile Oil, Dehydroacetic Acid, Glucose, Glycerin, Hawthron Stem Extract, Kaolin, Lavender Oil, Licorice Root Extract, Limonene, Linalool, Polysorbate 20, Sage Oil, Sorbitan Olivate, Manuka Oil, Thyme Oil, Water, Willow Bark Extract, Zinc Gluconate, Zinc Oxide
SULFUR
LICORICE EXTRACT
ZINC GLUCONATE
WILLOW BARK EXTRACT
CALCIUM CARBONATE
KAOLIN
ESSENTIAL OILS
A treatment mask formulated to combat and help prevent acne. It will reduce visible blemishes and leave skin looking clear, calm and even toned.
Results: Absorbs sebum and gently exfloiates to decongest acne-causing blocked pores. Reduces the appearance of pore size and redness. Soothes and calms. Lightens the appearance of pigmented acne scars.
REN's ClearCalm 3 system combats the 3 key causes of acne and blemishes: excess sebum: build up of dead skin cells; blemish-causing bacteria.... without harshness.
Skin type
Acne prone skin. Problem skin prone to breakouts and blemishes
REN CLEAN BIO ACTIVE SKINCARE
NO
PETROCHEMICALS, SULFATES, PARABENS, SYNTHETIC FRAGRANCE, SYNTHETIC COLOURS, T.E.A., D.E.A., GLYCOLS, SILICONES, PEGS, ET AL
SKINCARE AS IT SHOULD BE
DERMATOLOGIST TESTED AND APPROVED REN IS AGAINST ANIMAL TESTING
www.renskincare.com
Manufactured for:
REN Ltd, The Edison,
223-231
Old Marylebone Road,
London NW1 5QT
UK
Distributed by:
REN USA Inc,
250 Kennedy Drive,
Sayreville, New Jersey 08872
USA
Made in the UK
| CLEARCALM 3 ANTI-ACNE STARTER
sulfur cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Ren Ltd. (385613831) |