LAXATIVE REGULAR STRENGTH- sennosides tablet, coated
Walgreen Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Walgreens 44-347-Delisted
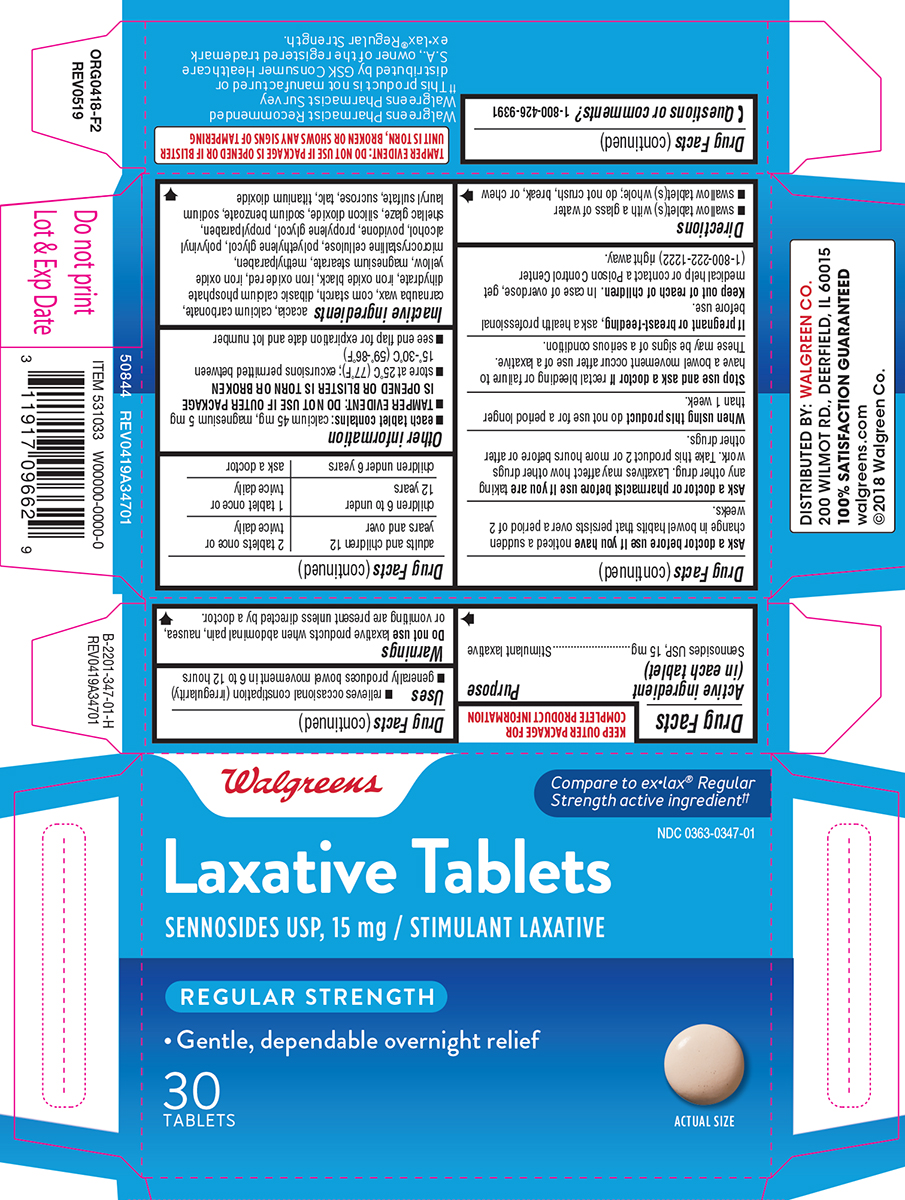
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
noticed a sudden change in bowel habits that persists over a period of 2 weeks.
Ask a doctor or pharmacist before use if you are
taking any other drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
Directions
- swallow tablet(s) with a glass of water
- swallow tablet(s) whole; do not crush, break, or chew
| adults and children 12 years and over | 2 tablets once or twice daily |
| children 6 to under 12 years | 1 tablet once or twice daily |
| children under 6 years | ask a doctor |
Other information
- each tablet contains: calcium 45 mg, magnesium 5 mg
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
acacia, calcium carbonate, carnauba wax, corn starch, dibasic calcium phosphate dihydrate, iron oxide black, iron oxide red, iron oxide yellow, magnesium stearate, methylparaben, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, propylene glycol, propylparaben, shellac glaze, silicon dioxide, sodium benzoate, sodium lauryl sulfate, sucrose, talc, titanium dioxide
Principal Display Panel
Walgreens
Compare to ex•lax® Regular Strength active ingredient††
NDC 0363-0347-01
Laxative Tablets
SENNOSIDES USP, 15 mg / STIMULANT LAXATIVE
REGULAR STRENGTH
• Gentle, dependable overnight relief
30 Tablets
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
††This product is not manufactured or distributed by GSK Consumer Healthcare S.A., owner of the registered trademark ex•lax® Regular Strength.
50844 REV0419A34701
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com ©2018 WalgreenCo.
44-347
| LAXATIVE
REGULAR STRENGTH
sennosides tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(0363-0347) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(0363-0347) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(0363-0347) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(0363-0347) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(0363-0347) | |