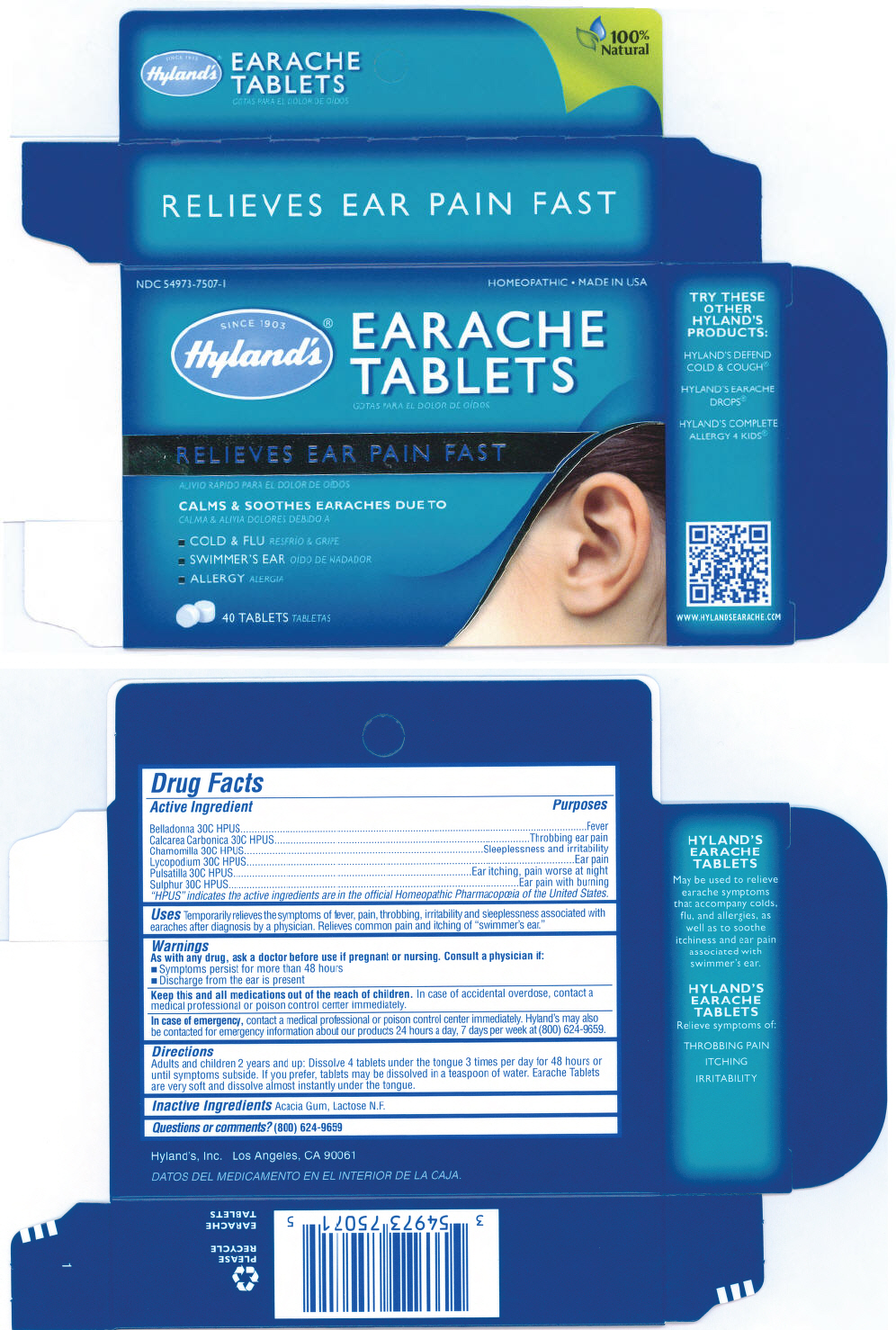
EARACHE- belladonna leaf, calcium carbonate, chamomile, lycopodium clavatum spore, pulsatilla patens, and sulfur tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active Ingredient | Purposes |
| "HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopœia of the United States. |
| Belladonna 30C HPUS | Fever |
| Calcarea Carbonica 30C HPUS | Throbbing ear pain |
| Chamomilla 30C HPUS | Sleeplessness and irritability |
| Lycopodium 30C HPUS | Ear pain |
| Pulsatilla 30C HPUS | Ear itching, pain worse at night |
| Sulphur 30C HPUS | Ear pain with burning |
Uses
Temporarily relieves the symptoms of fever, pain, throbbing, irritability and sleeplessness associated with earaches after diagnosis by a physician. Relieves common pain and itching of "swimmer's ear."
Warnings
As with any drug, ask a doctor before use if pregnant or nursing. Consult a physician if:
- Symptoms persist for more than 48 hours
- Discharge from the ear is present
Keep this and all medications out of the reach of children. In case of accidental overdose, contact a medical professional or poison control center immediately.
In case of emergency, contact a medical professional or poison control center immediately. Hyland's may also be contacted for emergency information about our products 24 hours a day, 7 days per week at (800) 624-9659.
Directions
Adults and children 2 years and up
Dissolve 4 tablets under the tongue 3 times per day for 48 hours or until symptoms subside. If you prefer, tablets may be dissolved in a teaspoon of water. Earache Tablets are very soft and dissolve almost instantly under the tongue.
Inactive Ingredients
Acacia Gum, Lactose N.F.
Questions or comments?
(800) 624-9659
PRINCIPAL DISPLAY PANEL - 4 x 10 Tablet Blister Pack Carton
NDC 54973-7507-1
HOMEOPATHIC • MADE IN USA
SINCE 1903
Hyland's®
EARACHE
TABLETS
RELIEVES EAR PAIN FAST
CALMS & SOOTHES EARACHES DUE TO
- COLD & FLU
- SWIMMER'S EAR
- ALLERGY
40 TABLETS