Label: ULTRAM- tramadol hcl tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 51655-185-20 - Packager: NORTHWIND PHARMACEUTICALS
- This is a repackaged label.
- Source NDC Code(s): 65162-627
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 14, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
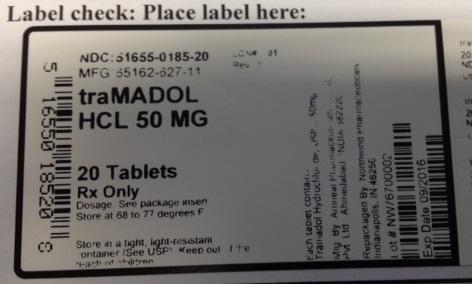
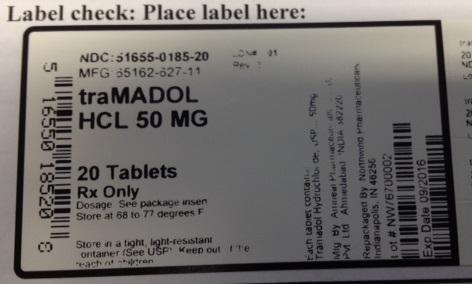
PRINCIPAL DISPLAY PANEL
NDC: 51655-0185-20
MFG: 65162-627-11
traMADOL HCL 50 MG
20 TABLETS RX ONLY
DOSAGE: SEE PACKAGE INSERT
STORE AT 68 TO 77 DEGREES F
STORE IN A TIGHT, LIGHT-RESISTANT CONTAINER. (SEE USP) KEEP OUT OF REACH OF CHILDREN
EACH TABLET CONTAINS TRAMADOL HYDROCHLORIDE USP 50 MG
MFG BY: AMNEAL PHARMACEUTICALS PVT LTD AHMEDABAD INDIA 382220
REPACKAGED BY NORTHWIND PHARMACEUTICALS INDIANAPOLIS, IN 46256
LOT# NW76700002
EXP DATE: 09/2016
- WARNINGS AND PRECAUTIONS
-
INGREDIENTS AND APPEARANCE
ULTRAM
tramadol hcl tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51655-185(NDC:65162-627) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 50 mg in 20 Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code AN627 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51655-185-20 20 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076003 02/13/2014 Labeler - NORTHWIND PHARMACEUTICALS (036986393) Registrant - NORTHWIND PHARMACEUTICALS (036986393) Establishment Name Address ID/FEI Business Operations NORTHWIND PHARMACEUTICALS 036986393 repack(51655-185)