CANKER AND COLD SORE- apis mellifica, arsenicum album, baptisia tinctoria, borax capsicum annuum, dulcamara, echinacea, mercurius corrosivus, nitricum acidum, pyrogenium, rhus toxicodendron, sulphur iodatum, thuja occ identalis, spray
Liddell Laboratories, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Canker and Cold Sore
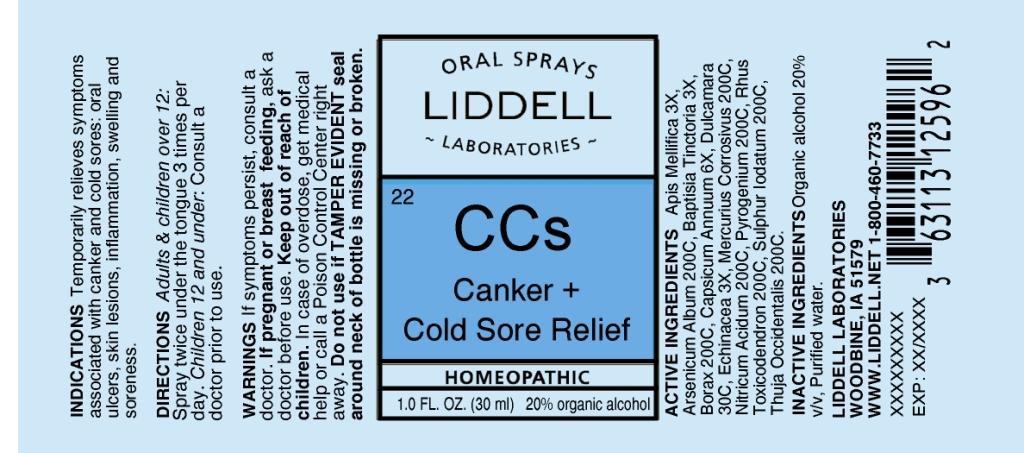
ACTIVE INGREDIENTS: Apis mellifica 3X, Arsenicum album 200C, Baptisia tinctoria 3X, Borax 200C, Capsicum annuum 6X, Dulcamara 30C, Echinacea 3X, Mercurius corrosivus 200C, Nitricum acidum 200C, Pyrogenium 200C, Rhus toxicodendron 200C, Sulphur iodatum 200C, Thuja occidentalis 200C.
INDICATIONS: Temporarily relieves symptoms associated with canker and cold sores: oral ulcers, skin lesions, inflammation, swelling and soreness.
WARNINGS: If symptoms persist, consult a doctor
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
DIRECTIONS: Adults and children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
| CANKER AND COLD SORE
apis mellifica, arsenicum album, baptisia tinctoria, borax capsicum annuum, dulcamara, echinacea, mercurius corrosivus, nitricum acidum, pyrogenium, rhus toxicodendron, sulphur iodatum, thuja occ identalis, spray |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Liddell Laboratories, Inc. (832264241) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Liddell Laboratories, Inc. | 832264241 | manufacture(50845-0120) | |