THEOPHYLLINE IN DEXTROSE- theophylline anhydrous injection, solution
Baxter Healthcare Corporation
----------
Theophylline in 5% Dextrose Injection, USP
in Plastic Container
VIAFLEX Plus Container
DESCRIPTION
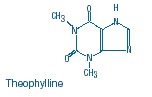
Theophylline in 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution of Theophylline, Anhydrous, USP in 5% Dextrose Injection. It contains no antimicrobial agents. Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione,3,7-dihydro-1,3-dimethyl-, and is represented by the following structural formula:
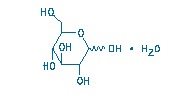
The molecular formula of anhydrous theophylline is C7H8N4O2 with a molecular weight of 180.17. Dextrose Hydrous, USP has the chemical name D-Glucose monohydrate and is represented by the following structural formula:
Theophylline in 5% Dextrose Injection, USP is intended for intravenous administration. Composition, osmolarity, pH and caloric content are shown in Table I.
|
||||||
|
Table I. |
||||||
|
Size (mL) |
Composition |
Osmolarity*
|
pH |
Caloric Content |
||
|
Theophylline Anhydrous, USP |
Dextrose Hydrous, USP |
|||||
|
200 mg Theophylline in 5% Dextrose Injection, USP |
50 |
200 |
50 |
275 |
4.5 |
170 |
|
100 |
200 |
50 |
263 |
4.5 |
170 |
|
|
400 mg Theophylline in 5% Dextrose Injection, USP |
100 |
400 |
50 |
275 |
4.5 |
170 |
|
250 |
400 |
50 |
261 |
4.5 |
170 |
|
|
500 |
400 |
50 |
257 |
4.5 |
170 |
|
|
1000 |
400 |
50 |
255 |
4.5 |
170 |
|
|
800 mg Theophylline in 5% Dextrose Injection, USP |
250 |
800 |
50 |
270 |
4.5 |
170 |
|
500 |
800 |
50 |
261 |
4.5 |
170 |
|
|
1000 |
800 |
50 |
257 |
4.5 |
170 |
|
This VIAFLEX Plus plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). VIAFLEX Plus on the container indicates the presence of a drug additive in a drug vehicle. The VIAFLEX Plus plastic container system utilizes the same container as the VIAFLEX plastic container system. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
CLINICAL PHARMACOLOGY
Mechanism of Action:
Theophylline has two distinct actions in the airways of patients with reversible obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., non-bronchodilator prophylactic effects). While the mechanisms of action of theophylline are not known with certainty, studies in animals suggest that bronchodilation is mediated by the inhibition of two isozymes of phosphodiesterase (PDE III and, to a lesser extent, PDE IV) while non-bronchodilator prophylactic actions are probably mediated through one or more different molecular mechanisms, that do not involve inhibition of PDE III or antagonism of adenosine receptors. Some of the adverse effects associated with theophylline appear to be mediated by inhibition of PDE III (e.g., hypotension, tachycardia, headache, and emesis) and adenosine receptor antagonism (e.g., alterations in cerebral blood flow).
Theophylline increases the force of contraction of diaphragmatic muscles. This action appears to be due to enhancement of calcium uptake through an adenosine-mediated channel.
Serum Concentration-Effect Relationship:
Bronchodilation occurs over the serum theophylline concentration range of 5-20 mcg/mL. Clinically important improvement in symptom control and pulmonary function has been found in most studies to require serum theophylline concentrations >10 mcg/mL. At serum theophylline concentrations >20 mcg/mL, both the frequency and severity of adverse reactions increase. In general, maintaining the average serum theophylline concentration between 10 and 15 mcg/mL will achieve most of the drug’s potential therapeutic benefit while minimizing the risk of serious adverse events.
Pharmacokinetics:
Overview
The pharmacokinetics of theophylline vary widely among similar patients and cannot be predicted by age, sex, body weight or other demographic characteristics. In addition, certain concurrent illnesses and alterations in normal physiology (see Table II) and co-administration of other drugs (see Table III) can significantly alter the pharmacokinetic characteristics of theophylline. Within-subject variability in metabolism has also been reported in some studies, especially in acutely ill patients. It is, therefore, recommended that serum theophylline concentrations be measured frequently in acutely ill patients receiving intravenous theophylline (e.g., at 24-hr intervals). More frequent measurements should be made during the initiation of therapy and in the presence of any condition that may significantly alter theophylline clearance (see PRECAUTIONS, Laboratory tests).
|
||
|
Table II. Mean and range of total body clearance and half-life of theophylline related to age and altered physiological states.* |
||
|
Population characteristics |
Half-life
|
|
|
Age |
||
|
Premature neonates | ||
|
postnatal age 3-15 days |
0.29 (0.09-0.49) |
30 (17-43) |
|
postnatal age 25-57 days |
0.64 (0.04-1.2) |
20 (9.4 -30.6) |
|
Term infants |
||
|
postnatal age 1-2 days |
NR§ |
25.7 (25-26.5) |
|
postnatal age 3-30 weeks |
NR§ |
11 (6-29) |
|
Children |
||
|
1-4 years |
1.7 (0.5-2.9) |
3.4 (1.2-5.6) |
|
4-12 years |
1.6 (0.8-2.4) |
NR§ |
|
13-15 years |
0.9 (0.48-1.3) |
NR§ |
|
6-17 years |
1.4 (0.2-2.6) |
3.7 (1.5-5.9) |
|
Adults (16-60 years) |
||
|
otherwise healthy | ||
|
nonsmoking asthmatics |
0.65 (0.27-1.03) |
8.7 (6.1-12.8) |
|
Elderly (>60 years) |
||
|
nonsmokers with normal cardiac, liver, and renal function |
0.41 (0.21-0.61) |
9.8 (1.6-18) |
|
Concurrent illness or |
||
|
Acute pulmonary edema |
0.33¶ (0.07-2.45) |
19¶ (3.1-82) |
|
COPD->60 years, stable | ||
|
nonsmoker >1 year |
0.54 (0.44-0.64) |
11 (9.4- 12.6) |
|
COPD with cor pulmonale |
0.48 (0.08-0.88) |
NR§ |
|
Cystic fibrosis (14-28 years) |
1.25 (0.31-2.2) |
6.0 (1.8-10.2) |
|
Fever associated with acute viral respiratory illness (children 9-15 years) |
NR§ |
7.0 (1.0-13) |
|
Liver disease - |
||
|
cirrhosis |
0.31¶ (0.1-0.7) |
32¶ (10-56) |
|
acute hepatitis |
0.35 (0.25-0.45) |
19.2 (16.6-21.8) |
|
cholestasis |
0.65 (0.25-1.45) |
14.4 (5.7-31.8) |
|
Pregnancy - |
||
|
1st trimester |
NR§ |
8.5 (3.1-13.9) |
|
2nd trimester |
NR§ |
8.8 (3.8-13.8) |
|
3rd trimester |
NR§ |
13.0 (8.4-17.6) |
|
Sepsis with multi-organ failure |
0.47 (0.19-1.9) |
18.8 (6.3-24.1) |
|
Thyroid disease - |
||
|
hypothyroid |
0.38 (0.13-0.57) |
11.6 (8.2-25) |
|
hyperthyroid |
0.8 (0.68-0.97) |
4.5 (3.7-5.6) |
Distribution
Once theophylline enters the systemic circulation, about 40% is bound to plasma protein, primarily albumin. Unbound theophylline distributes throughout body water, but distributes poorly into body fat. The apparent volume of distribution of theophylline is approximately 0.45 L/kg (range 0.3-0.7 L/kg) based on ideal body weight. Theophylline passes freely across the placenta, into breast milk and into the cerebrospinal fluid (CSF). Saliva theophylline concentrations approximate unbound serum concentrations, but are not reliable for routine or therapeutic monitoring unless special techniques are used. An increase in the volume of distribution of theophylline, primarily due to reduction in plasma protein binding, occurs in premature neonates, patients with hepatic cirrhosis, uncorrected acidemia, the elderly and in women during the third trimester of pregnancy. In such cases, the patient may show signs of toxicity at total (bound + unbound) serum concentrations of theophylline in the therapeutic range (10-20 mcg/mL) due to elevated concentrations of the pharmacologically active unbound drug. Similarly, a patient with decreased theophylline binding may have a sub-therapeutic total drug concentration while the pharmacologically active unbound concentration is in the therapeutic range. If only total serum theophylline concentration is measured, this may lead to an unnecessary and potentially dangerous dose increase. In patients with reduced protein binding, measurement of unbound serum theophylline concentration provides a more reliable means of dosage adjustment than measurement of total serum theophylline concentration. Generally, concentrations of unbound theophylline should be maintained in the range of 6-12 mcg/mL.
Metabolism
In adults and children beyond one year of age, approximately 90% of the dose is metabolized in the liver. Biotransformation takes place through de-methylation to 1-methylxanthine and 3-methylxanthine and hydroxylation to 1,3-dimethyluric acid.
1-methylxanthine is further hydroxylated, by xanthine oxidase, to 1-methyluric acid. About 6% of a theophylline dose is N-methylated to caffeine. Theophylline de-methylation to 3-methylxanthine is catalyzed by cytochrome P-450 1A2, while cytochromes P-450 2E1 and P-450 3A3 catalyze the hydroxylation to 1,3-dimethyluric acid. De-methylation to 1-methylxanthine appears to be catalyzed either by cytochrome P-450 1A2 or a closely related cytochrome. In neonates, the N-de-methylation pathway is absent while the function of the hydroxylation pathway is markedly deficient. The activity of these pathways slowly increases to maximal levels by one year of age.
Caffeine and 3-methylxanthine are the only theophylline metabolites with pharmacologic activity. 3-methylxanthine has approximately one tenth the pharmacologic activity of theophylline and serum concentrations in adults with normal renal function are <1 mcg/mL. In patients with end-stage renal disease, 3-methylxanthine may accumulate to concentrations that approximate the unmetabolized theophylline concentration. Caffeine concentrations are usually undetectable in adults regardless of renal function. In neonates, caffeine may accumulate to concentrations that approximate the unmetabolized theophylline concentration and thus, exert a pharmacologic effect.
Both the N-de-methylation and hydroxylation pathways of theophylline biotransformation are capacity-limited. Due to the wide intersubject variability of the rate of theophylline metabolism, non-linearity of elimination may begin in some patients at serum theophylline concentrations <10 mcg/mL. Since this non-linearity results in more than proportional changes in serum theophylline concentrations with changes in dose, it is advisable to make increases or decreases in dose in small increments in order to achieve desired changes in serum theophylline concentrations (see DOSAGE AND ADMINISTRATION, Table VII). Accurate prediction of dose-dependency of theophylline metabolism in patients a priori is not possible, but patients with very high initial clearance rates (i.e., low steady-state serum theophylline concentrations at above average doses) have the greatest likelihood of experiencing large changes in serum theophylline concentration in response to dosage changes.
Excretion
In neonates, approximately 50% of the theophylline dose is excreted unchanged in the urine. Beyond the first three months of life, approximately 10% of the theophylline dose is excreted unchanged in the urine. The remainder is excreted in the urine mainly as 1,3-dimethyluric acid (35-40%), 1-methyluric acid (20-25%) and 3-methylxanthine (15-20%). Since little theophylline is excreted unchanged in the urine and since active metabolites of theophylline (i.e., caffeine, 3-methylxanthine) do not accumulate to clinically significant levels even in the face of end-stage renal disease, no dosage adjustment for renal insufficiency is necessary in adults and children >3 months of age. In contrast, the large fraction of the theophylline dose excreted in the urine as unchanged theophylline and caffeine in neonates requires careful attention to dose reduction and frequent monitoring of serum theophylline concentrations in neonates with reduced renal function (See WARNINGS).
Serum Concentrations at Steady-State
In a patient who has received no theophylline in the previous 24 hours, a loading dose of intravenous theophylline of 4.6 mg/kg calculated on the basis of ideal body weight and administered over 30 minutes, on average, will produce a maximum post-distribution serum concentration of 10 mcg/mL with a range of 6-16 mcg/mL. In nonsmoking adults, initiation of a constant intravenous theophylline infusion of 0.4 mg/kg/hr at the completion of the loading dose, on average, will result in a steady-state concentration of 10 mcg/mL with a range of 7-26 mcg/mL. The mean and range of steady-state serum concentrations are similar when the average child (age 1 to 9 years) is given a loading dose of 4.6 mg/kg theophylline followed by a constant intravenous infusion of 0.8 mg/kg/hr. (See DOSAGE AND ADMINISTRATION)
Special Populations (See Table II for mean clearance and half-life values)
Geriatric
The clearance of theophylline is decreased by an average of 30% in healthy elderly adults (>60 yrs) compared to healthy young adults. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in elderly patients (see WARNINGS).
Pediatrics
The clearance of theophylline is very low in neonates (see WARNINGS). Theophylline clearance reaches maximal values by one year of age, remains relatively constant until about 9 years of age and then slowly decreases by approximately 50% to adult values at about age 16. Renal excretion of unchanged theophylline in neonates amounts to about 50% of the dose, compared to about 10% in children older than three months and in adults. Careful attention to dosage selection and monitoring of serum theophylline concentrations are required in pediatric patients (see WARNINGS and DOSAGE AND ADMINISTRATION).
Gender
Gender differences in theophylline clearance are relatively small and unlikely to be of clinical significance. Significant reduction in theophylline clearance, however, has been reported in women on the 20th day of the menstrual cycle and during the third trimester of pregnancy.
Renal Insufficiency
Only a small fraction, e.g., about 10%, of the administered theophylline dose is excreted unchanged in the urine of children greater than three months of age and adults. Since little theophylline is excreted unchanged in the urine and since active metabolites of theophylline (i.e., caffeine, 3-methylxanthine) do not accumulate to clinically significant levels even in the face of end-stage renal disease, no dosage adjustment for renal insufficiency is necessary in adults and children >3 months of age. In contrast, approximately 50% of the administered theophylline dose is excreted unchanged in the urine in neonates. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in neonates with decreased renal function (see WARNINGS).
Hepatic Insufficiency
Theophylline clearance is decreased by 50% or more in patients with hepatic insufficiency (e.g., cirrhosis, acute hepatitis, cholestasis). Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with reduced hepatic function (see WARNINGS).
Congestive Heart Failure (CHF)
Theophylline clearance is decreased by 50% or more in patients with CHF. The extent of reduction in theophylline clearance in patients with CHF appears to be directly correlated to the severity of the cardiac disease. Since theophylline clearance is independent of liver blood flow, the reduction in clearance appears to be due to impaired hepatocyte function rather than reduced perfusion. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with CHF (see WARNINGS).
Smokers
Tobacco and marijuana smoking appears to increase the clearance of theophylline by induction of metabolic pathways. Theophylline clearance has been shown to increase by approximately 50% in young adult tobacco smokers and by approximately 80% in elderly tobacco smokers compared to nonsmoking subjects. Passive smoke exposure has also been shown to increase theophylline clearance by up to 50%. Abstinence from tobacco smoking for one week causes a reduction of approximately 40% in theophylline clearance. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients who stop smoking (see WARNINGS). Use of nicotine gum has been shown to have no effect on theophylline clearance.
Fever
Fever, regardless of its underlying cause, can decrease the clearance of theophylline. The magnitude and duration of the fever appear to be directly correlated to the degree of decrease of theophylline clearance. Precise data are lacking, but a temperature of 39ºC (102ºF) for at least 24 hours is probably required to produce a clinically significant increase in serum theophylline concentrations. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with sustained fever (see WARNINGS).
Miscellaneous
Other factors associated with decreased theophylline clearance include the third trimester of pregnancy, sepsis with multiple organ failure, and hypothyroidism. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with any of these conditions (see WARNINGS). Other factors associated with increased theophylline clearance include hyperthyroidism and cystic fibrosis.
Clinical Studies:
Inhaled beta-2 selective agonists and systemically administered corticosteroids are the treatments of first choice for management of acute exacerbations of asthma. The results of controlled clinical trials on the efficacy of adding intravenous theophylline to inhaled beta-2 selective agonists and systemically administered corticosteroids in the management of acute exacerbations of asthma have been conflicting. Most studies in patients treated for acute asthma exacerbations in an emergency department have shown that addition of intravenous theophylline does not produce greater bronchodilation and increases the risk of adverse effects. In contrast, other studies have shown that addition of intravenous theophylline is beneficial in the treatment of acute asthma exacerbations in patients requiring hospitalization, particularly in patients who are not responding adequately to inhaled beta-2 selective agonists.
In patients with chronic obstructive pulmonary disease (COPD), clinical studies have shown that theophylline decreases dyspnea, air trapping, the work of breathing, and improves contractility of diaphragmatic muscles with little or no improvement in pulmonary function measurements.
INDICATIONS AND USAGE
Intravenous theophylline is indicated as an adjunct to inhaled beta-2 selective agonists and systemically administered corticosteroids for the treatment of acute exacerbations of the symptoms and reversible airflow obstruction associated with asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
CONTRAINDICATIONS
Theophylline in 5% Dextrose Injection, USP is contraindicated in patients with a history of hypersensitivity to theophylline or other components in the product. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
WARNINGS
Concurrent Illness
Theophylline should be used with extreme caution in patients with the following clinical conditions due to the increased risk of exacerbation of the concurrent condition:
Active peptic ulcer disease
Seizure disorders
Cardiac arrhythmias (not including bradyarrhythmias)
Conditions That Reduce Theophylline Clearance:
There are several readily identifiable causes of reduced theophylline clearance. If the infusion rate is not appropriately reduced in the presence of these risk factors, severe and potentially fatal theophylline toxicity can occur. Careful consideration must be given to the benefits and risks of theophylline use and the need for more intensive monitoring of serum theophylline concentrations in patients with the following risk factors:
Age
Neonates (term and premature)
Children <1 year
Elderly (>60 years)
Concurrent Diseases
Acute pulmonary edema
Congestive heart failure
Cor pulmonale
Fever; ≥102º for 24 hours or more; or lesser temperature elevations for longer periods
Hypothyroidism
Liver disease; cirrhosis, acute hepatitis
Reduced renal function in infants <3 months of age
Sepsis with multi-organ failure
Shock
Cessation of Smoking
Drug Interactions Adding a drug that inhibits theophylline metabolism (e.g., cimetidine, erythromycin, tacrine) or stopping a concurrently administered drug that enhances theophylline metabolism (e.g., carbamazepine, rifampin). (See PRECAUTIONS, Drug Interactions, Table III).
When Signs or Symptoms of Theophylline Toxicity Are Present:
Whenever a patient receiving theophylline develops nausea or vomiting, particularly repetitive vomiting, or other signs or symptoms consistent with theophylline toxicity (even if another cause may be suspected), the intravenous administration should be stopped and a serum theophylline concentration measured immediately.
Dosage Increases:
Increases in the dose of intravenous theophylline should not be made in response to an acute exacerbation of symptoms unless the steady-state serum theophylline concentration is <10 mcg/mL.
As the rate of theophylline clearance may be dose-dependent (i.e., steady-state serum concentrations may increase disproportionately to the increase in dose), an increase in dose based upon a sub-therapeutic serum concentration measurement should be conservative. In general, limiting infusion rate increases to about 25% of the previous infusion rate will reduce the risk of unintended excessive increases in serum theophylline concentration (see DOSAGE AND ADMINISTRATION, Table VII).
Solutions containing dextrose should not be administered simultaneously through the same administration set as blood, as this may result in pseudoagglutination or hemolysis.
The intravenous administration of solutions may cause fluid overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
PRECAUTIONS
General:
Careful consideration of the various interacting drugs and physiologic conditions that can alter theophylline clearance and require dosage adjustment should occur prior to initiation of theophylline therapy and prior to increases in theophylline dose (see WARNINGS).
Monitoring Serum Theophylline Concentrations:
Serum theophylline concentration measurements are readily available and should be used to determine whether the dosage is appropriate. Specifically, the serum theophylline concentration should be measured as follows:
- 1.
- Before making a dose increase to determine whether the serum concentration is sub-therapeutic in a patient who continues to be symptomatic.
- 2.
- Whenever signs or symptoms of theophylline toxicity are present.
- 3.
- Whenever there is a new illness, worsening of an existing concurrent illness or a change in the patient’s treatment regimen that may alter theophylline clearance (e.g., fever >102º F sustained for ≥24 hours, hepatitis, or drugs listed in Table III are added or discontinued).
In patients who have received no theophylline in the previous 24 hours, a serum concentration should be measured 30 minutes after completion of the intravenous loading dose to determine whether the serum concentration is <10 mcg/mL indicating the need for an additional loading dose or >20 mcg/mL indicating the need to delay starting the constant IV infusion. Once the infusion is begun, a second measurement should be obtained after one expected half-life (e.g., approximately 4 hours in children age 1 to 9 years and 8 hours in nonsmoking adults; See Table II for the expected half-life in additional patient populations). The second measurement should be compared to the first to determine the direction in which the serum concentration has changed. The infusion rate can then be adjusted before steady-state is reached in an attempt to prevent an excessive or sub-therapeutic theophylline concentration from being achieved. If a patient has received theophylline in the previous 24 hours, the serum concentration should be measured before administering an intravenous loading dose to make sure that it is safe to do so. If a loading dose is not indicated (i.e., the serum theophylline concentration is ≥10 mcg/mL), a second measurement should be obtained as above at the appropriate time after starting the intravenous infusion. If, on the other hand, a loading dose is indicated (see DOSAGE AND ADMINISTRATION for guidance on selection of the appropriate loading dose), a second blood sample should be obtained after the loading dose and a third sample should be obtained one expected half-life after starting the constant infusion to determine the direction in which the serum concentration has changed.
Once the above procedures related to initiation of intravenous theophylline infusion have been completed, subsequent serum samples for determination of theophylline concentration should be obtained at 24-hour intervals for the duration of the infusion. The theophylline infusion rate should be increased or decreased as appropriate based on the serum theophylline levels. When signs or symptoms of theophylline toxicity are present, the intravenous infusion should be stopped and a serum sample for theophylline concentration should be obtained as soon as possible, analyzed immediately, and the result reported to the clinician without delay. In patients in whom decreased serum protein binding is suspected (e.g., cirrhosis, women during the third trimester of pregnancy), the concentration of unbound theophylline should be measured and the dosage adjusted to achieve an unbound concentration of 6-12 mcg/mL.
Saliva concentrations of theophylline cannot be used reliably to adjust dosage without special techniques.
Effects on Laboratory Tests:
As a result of its pharmacological effects, theophylline at serum concentrations within the 10-20 mcg/mL range modestly increases plasma glucose (from a mean of 88 mg% to 98 mg%), uric acid (from a mean of 4 mg/dl to 6 mg/dl), free fatty acids (from a mean of 451 µεq/l to 800 µεq/l), total cholesterol (from a mean of 140 vs. 160 mg/dl), HDL (from a mean of 36 to 50 mg/dl), HDL/LDL ratio (from a mean of 0.5 to 0.7), and urinary free cortisol excretion (from a mean of 44 to 63 mcg/24 hr). Theophylline at serum concentrations within the 10-20 mcg/mL range may also transiently decrease serum concentrations of triiodothyronine (144 before, 131 after one week and 142 ng/dl after 4 weeks of theophylline). The clinical importance of these changes should be weighed against the potential therapeutic benefit of theophylline in individual patients.
Drug Interactions:
Theophylline interacts with a wide variety of drugs. The interaction may be pharmacodynamic, i.e., alterations in the therapeutic response to theophylline or another drug or occurrence of adverse effects without a change in serum theophylline concentration. More frequently, however, the interaction is pharmacokinetic, i.e., the rate of theophylline clearance is altered by another drug resulting in increased or decreased serum theophylline concentrations. Theophylline only rarely alters the pharmacokinetics of other drugs.
The drugs listed in Table III have the potential to produce clinically significant pharmacodynamic or pharmacokinetic interactions with theophylline. The information in the “Effect” column of Table III assumes that the interacting drug is being added to a steady-state theophylline regimen. If theophylline is being initiated in a patient who is already taking a drug that inhibits theophylline clearance (e.g., cimetidine, erythromycin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be smaller. Conversely, if theophylline is being initiated in a patient who is already taking a drug that enhances theophylline clearance (e.g., rifampin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be larger. Discontinuation of a concomitant drug that increases theophylline clearance will result in accumulation of theophylline to potentially toxic levels, unless the theophylline dose is appropriately reduced. Discontinuation of a concomitant drug that inhibits theophylline clearance will result in decreased serum theophylline concentrations, unless the theophylline dose is appropriately increased.
The drugs listed in Table IV have either been documented not to interact with theophylline or do not produce a clinically significant interaction (i.e., <15% change in theophylline clearance).
The listings of drugs in Tables III and IV are current as of September 1, 1995. New interactions are continuously being reported for theophylline, especially with new chemical entities. The clinician should not assume that a drug does not interact with theophylline if it is not listed in Table III. Before addition of a newly available drug in a patient receiving theophylline, the package insert of the new drug and/or the medical literature should be consulted to determine if an interaction between the new drug and theophylline has been reported.
|
||
|
Table III. Clinically significant drug interactions with theophylline.* |
||
|
Drug |
Type of Interaction |
Effect† |
|
Adenosine |
Theophylline blocks adenosine receptors. |
Higher doses of adenosine may be required to achieve desired effect. |
|
Alcohol |
A single large dose of alcohol (3 mL/kg of whiskey) decreases theophylline clearance for up to 24 hours. |
30% increase |
|
Allopurinol |
Decreases theophylline clearance at allopurinol doses ≥600 mg/day. |
25% increase |
|
Aminoglutethimide |
Increases theophylline clearance by induction of microsomal enzyme activity. |
25% decrease |
|
Carbamazepine |
Similar to aminoglutethimide. |
30% decrease |
|
Cimetidine |
Decreases theophylline clearance by inhibiting cytochrome P450 1A2. |
70% increase |
|
Ciprofloxacin |
Similar to cimetidine. |
40% increase |
|
Clarithromycin |
Similar to erythromycin. |
25% increase |
|
Diazepam |
Benzodiazepines increase CNS concentrations of adenosine, a potent CNS depressant, while theophylline blocks adenosine receptors. |
Larger diazepam doses may be required to produce desired level of sedation. Discontinuation of theophylline without reduction of diazepam dose may result in respiratory depression. |
|
Disulfiram |
Decreases theophylline clearance by inhibiting hydroxylation and demethylation. |
50% increase |
|
Enoxacin |
Similar to cimetidine. |
300% increase |
|
Ephedrine |
Synergistic CNS effects. |
Increased frequency of nausea, nervousness, and insomnia. |
|
Erythromycin |
Erythromycin metabolite decreases theophylline clearance by inhibiting cytochrome P450 3A3. |
35% increase. Erythromycin steady-state serum concentrations decrease by a similar amount. |
|
Estrogen |
Estrogen-containing oral contraceptives decrease theophylline clearance in a dose-dependent fashion. The effect of progesterone on theophylline clearance is unknown. |
30% increase |
|
Flurazepam |
Similar to diazepam. |
Similar to diazepam. |
|
Fluvoxamine |
Similar to cimetidine. |
Similar to cimetidine. |
|
Halothane |
Halothane sensitizes the myocardium to catecholamines, theophylline increases release of endogenous catecholamines. |
Increased risk of ventricular arrhythmias. |
|
Interferon, human recombinant alpha-A |
Decreases theophylline clearance. |
100% increase |
|
Isoproterenol (IV) |
Increases theophylline clearance. |
20% decrease |
|
Ketamine |
Pharmacologic |
May lower theophylline seizure threshold. |
|
Lithium |
Theophylline increases renal lithium clearance. |
Lithium dose required to achieve a therapeutic serum concentration increased an average of 60%. |
|
Lorazepam |
Similar to diazepam. |
Similar to diazepam. |
|
Methotrexate (MTX) |
Decreases theophylline clearance. |
20% increase after low dose MTX, higher dose MTX may have a greater effect. |
|
Mexiletine |
Similar to disulfiram. |
80% increase |
|
Midazolam |
Similar to diazepam. |
Similar to diazepam. |
|
Moricizine |
Increases theophylline clearance. |
25% decrease |
|
Pancuronium |
Theophylline may antagonize non-depolarizing neuromuscular blocking effects; possibly due to phosphodiesterase inhibition. |
Larger dose of pancuronium may be required to achieve neuromuscular blockade. |
|
Pentoxifylline |
Decreases theophylline clearance. |
30% increase |
|
Phenobarbital (PB) |
Similar to aminoglutethimide. |
25% decrease after two weeks of concurrent PB. |
|
Phenytoin |
Phenytoin increases theophylline clearance by increasing microsomal enzyme activity. Theophylline decreases phenytoin absorption. |
Serum theophylline and phenytoin concentrations decrease about 40%. |
|
Propafenone |
Decreases theophylline clearance and pharmacologic interaction. |
40% increase. Beta-2 blocking effect may decrease efficacy of theophylline. |
|
Propranolol |
Similar to cimetidine and pharmacologic interaction. |
100% increase. Beta-2 blocking effect may decrease efficacy of theophylline. |
|
Rifampin |
Increases theophylline clearance by increasing cytochrome P450 1A2 and 3A3 activity. |
20-40% decrease |
|
Sulfinpyrazone |
Increases theophylline clearance by increasing demethylation and hydroxylation. Decreases renal clearance of theophylline. |
20% decrease |
|
Tacrine |
Similar to cimetidine, also increases renal clearance of theophylline. |
90% increase |
|
Thiabendazole |
Decreases theophylline clearance. |
190% increase |
|
Ticlopidine |
Decreases theophylline clearance. |
60% increase |
|
Troleandomycin |
Similar to erythromycin. |
33-100% increase depending on troleandomycin dose. |
|
Verapamil |
Similar to disulfiram. |
20% increase |
|
|
|
Table IV. Drugs that have been documented not to interact with theophylline or drugs that produce no clinically significant interaction with theophylline.* |
|
|
albuterol, systemic and inhaled |
lomefloxacin |
|
amoxicillin |
medroxyprogesterone |
|
ampicillin, with or without sulbactam |
methylprednisolone |
|
atenolol |
metoprolol |
|
azithromycin |
nadolol |
|
caffeine, dietary ingestion |
nifedipine |
|
cefaclor |
norfloxacin |
|
co-trimoxazole |
ofloxacin |
|
diltiazem |
ranitidine |
|
dirithromycin |
rifabutin |
|
enflurane |
roxithromycin |
|
famotidine |
sorbitol (purgative doses do not inhibit theophylline absorption) |
|
felodipine |
|
|
finasteride |
|
|
hydrocortisone |
|
|
insoflurane |
sucralfate |
|
isoniazid |
terbutaline, systemic |
|
isradipine |
terfenadine |
|
influenza vaccine |
tetracycline |
|
ketoconazole |
tocainide |
The Effect of Other Drugs on Theophylline Serum Concentration Measurements:
Most serum theophylline assays in clinical use are immunoassays which are specific for theophylline. Other xanthines such as caffeine, dyphylline, and pentoxifylline are not detected by these assays. Some drugs (e.g., cefazolin, cephalothin), however, may interfere with certain HPLC techniques. Caffeine and xanthine metabolites in neonates or patients with renal dysfunction may cause the reading from some dry reagent office methods to be higher than the actual serum theophylline concentration.
Carcinogenesis and Mutagenesis and Impairment of Fertility:
Long term carcinogenicity studies have been carried out in mice (oral doses 30-150 mg/kg) and rats (oral doses 5-75 mg/kg). Results are pending.
Theophylline has been studied in Ames salmonella, in vivo and in vitro cytogenetics, micronucleus and Chinese hamster ovary test systems and has not been shown to be genotoxic.
In a 14 week continuous breeding study, theophylline, administered to mating pairs of B6C3F1 mice at oral doses of 120, 270 and 500 mg/kg (approximately 1.0-3.0 times the human dose on a mg/m2 basis) impaired fertility, as evidenced by decreases in the number of live pups per litter, decreases in the mean number of litters per fertile pair, and increases in the gestation period at the high dose as well as decreases in the proportion of pups born alive at the mid and high dose. In 13 week toxicity studies, theophylline was administered to F344 rats and B6C3F1 mice at oral doses of 40-300 mg/kg (approximately 2.0 times the human dose on a mg/m2 basis). At the high dose, systemic toxicity was observed in both species including decreases in testicular weight.
Pregnancy
Pregnancy Category C
Teratogenic Effects
There are no adequate and well-controlled studies in pregnant women. In animal reproduction studies, maternal doses of theophylline less than one to two times the maximum recommended oral dose in humans caused fetal harm, including fetal malformations. Asthma is a serious and potentially life-threatening condition. Poorly controlled asthma during pregnancy is associated with adverse outcomes for mother and fetus. Theophylline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Population-based studies and post-marketing adverse event reporting of theophylline use during human pregnancy have not demonstrated an increased risk of major congenital anomalies. However, most studies were not large enough to detect a less than two fold increase in risk for congenital anomalies. Post-marketing data are reported voluntarily and do not always reliably estimate the frequency of particular adverse outcomes.
In animal reproduction studies, theophylline produced teratogenic effects when pregnant mice, rats and rabbits were dosed during the period of organogenesis.
In mice, a single intraperitoneal dose at and above 100 mg/kg (approximately equal to the maximum recommended oral dose for adults on a mg/m2 basis) produced cleft palate and digital abnormalities. Micromelia, micrognathia, clubfoot, subcutaneous hematoma, open eyelids, and embryolethality were observed at doses approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis.
In rats dosed from conception through organogenesis, an oral dose of 150 mg/kg/day (approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis) produced digital abnormalities. Embryolethality occurred at a subcutaneous dose of 200 mg/kg/day (approximately 4 times the maximum recommended oral dose for adults on a mg/m2 basis). In rabbits dosed intravenously throughout organogenesis with 60 mg/kg/day (approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis), caused cleft palate and was embryolethal. This dose was maternally toxic as one doe died and clinical signs of toxicity occurred in others. Doses at and above 15 mg/kg/day (less than the maximum recommended oral dose for adults on a mg/m2 basis) increased the incidence of skeletal variations.
Nursing Mothers:
Theophylline is excreted into breast milk and may cause irritability or other signs of mild toxicity in nursing human infants. The concentration of theophylline in breast milk is about equivalent to the maternal serum concentration. An infant ingesting a liter of breast milk containing 10-20 mcg/mL of theophylline a day is likely to receive 10-20 mg of theophylline per day. Serious adverse effects in the infant are unlikely unless the mother has toxic serum theophylline concentrations.
Pediatric Use:
Theophylline is safe and effective for the approved indications in pediatric patients (See, INDICATIONS AND USAGE). The constant infusion rate of intravenous theophylline must be selected with caution in children since the rate of theophylline clearance is highly variable across the age range of neonates to adolescents (see CLINICAL PHARMACOLOGY, Table II, WARNINGS, and DOSAGE AND ADMINISTRATION, Table VI). Due to the immaturity of theophylline metabolic pathways in children under the age of one year, particular attention to dosage selection and frequent monitoring of serum theophylline concentrations are required when theophylline is prescribed to pediatric patients in this age group.
Geriatric Use:
Elderly patients are at significantly greater risk of experiencing serious toxicity from theophylline than younger patients due to pharmacokinetic and pharmacodynamic changes associated with aging. Theophylline clearance is reduced in patients greater than 60 years of age, resulting in increased serum theophylline concentrations in response to a given theophylline infusion rate. Protein binding may be decreased in the elderly resulting in a larger proportion of the total serum theophylline concentration in the pharmacologically active unbound form. Elderly patients also appear to be more sensitive to the toxic effects of theophylline after chronic overdosage than younger patients. For these reasons, the maximum infusion rate of theophylline in patients greater than 60 years of age ordinarily should not exceed 17 mg/hr unless the patient continues to be symptomatic and the peak steady-state serum theophylline concentration is <10 mcg/mL (see DOSAGE AND ADMINISTRATION). Theophylline infusion rates greater than 17 mg/hr should be prescribed with caution in elderly patients.
Do not administer unless solution is clear and seal is intact.
ADVERSE REACTIONS
Adverse reactions associated with theophylline are generally mild when peak serum theophylline concentrations are <20 mcg/mL and mainly consist of transient caffeine-like adverse effects such as nausea, vomiting, headache, and insomnia. When serum theophylline concentrations exceed 20 mcg/mL, however, theophylline produces a wide range of adverse reactions including persistent vomiting, cardiac arrhythmias, and intractable seizures which can be lethal (see OVERDOSAGE).
Other adverse reactions that have been reported at serum theophylline concentrations <20 mcg/mL include diarrhea, irritability, restlessness, fine skeletal muscle tremors, and transient diuresis. In patients with hypoxia secondary to COPD, multifocal atrial tachycardia and flutter have been reported at serum theophylline concentrations ≥15 mcg/mL. There have been a few isolated reports of seizures at serum theophylline concentrations <20 mcg/mL in patients with an underlying neurological disease or in elderly patients. The occurrence of seizures in elderly patients with serum theophylline concentrations <20 mcg/mL may be secondary to decreased protein binding resulting in a larger proportion of the total serum theophylline concentration in the pharmacologically active unbound form. The clinical characteristics of the seizures reported in patients with serum theophylline concentrations <20 mcg/mL have generally been milder than seizures associated with excessive serum theophylline concentrations resulting from an overdose (i.e., they have generally been transient, often stopped without anticonvulsant therapy, and did not result in neurological residua).
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
General:
The chronicity and pattern of theophylline overdosage significantly influences clinical manifestations of toxicity, management and outcome. There are two common presentations: (1) acute overdose, i.e., infusion of an excessive loading dose or excessive maintenance infusion rate for less than 24 hours, and (2) chronic overdosage, i.e., excessive maintenance infusion rate for greater than 24 hours. The most common causes of chronic theophylline overdosage include clinician prescribing of an excessive dose or a normal dose in the presence of factors known to decrease the rate of theophylline clearance, and increasing the dose in response to an exacerbation of symptoms without first measuring the serum theophylline concentration to determine whether a dose increase is safe.
Several studies have described the clinical manifestations of theophylline overdose following oral administration and attempted to determine the factors that predict life-threatening toxicity. In general, patients who experience an acute overdose are less likely to experience seizures than patients who have experienced a chronic overdosage, unless the peak serum theophylline concentration is >100 mcg/mL. After a chronic overdosage, generalized seizures, life-threatening cardiac arrhythmias, and death may occur at serum theophylline concentrations >30 mcg/mL. The severity of toxicity after chronic overdosage is more strongly correlated with the patient’s age than the peak serum theophylline concentration; patients >60 years are at the greatest risk for severe toxicity and mortality after a chronic overdosage. Pre-existing or concurrent disease may also significantly increase the susceptibility of a patient to a particular toxic manifestation, e.g., patients with neurologic disorders have an increased risk of seizures and patients with cardiac disease have an increased risk of cardiac arrhythmias for a given serum theophylline concentration compared to patients without the underlying disease.
The frequency of various reported manifestations of oral theophylline overdose according to the mode of overdose are listed in Table V.
|
||||
|
Table V. Manifestations of theophylline toxicity.* |
||||
|
Sign/Symptom |
Percentage of patients reported with sign or symptom |
|||
|
Acute Overdose |
Chronic Overdosage |
|||
|
Study 1 (n=157) |
Study 2 (n=14) |
Study 1 (n=92) |
Study 2 (n=102) |
|
|
Asymptomatic |
NR† |
0 |
NR† |
6 |
|
Gastrointestinal | ||||
|
Vomiting |
73 |
93 |
30 |
61 |
|
Abdominal Pain |
NR† |
21 |
NR† |
12 |
|
Diarrhea |
NR† |
0 |
NR† |
14 |
|
Hematemesis |
NR† |
0 |
NR† |
2 |
|
Metabolic/Other | ||||
|
Hypokalemia |
85 |
79 |
44 |
43 |
|
Hyperglycemia |
98 |
NR† |
18 |
NR† |
|
Acid/base disturbance |
34 |
21 |
9 |
5 |
|
Rhabdomyolysis |
NR† |
7 |
NR† |
0 |
|
Cardiovascular | ||||
|
Sinus tachycardia |
100 |
86 |
100 |
62 |
|
Other supraventricular tachycardias |
2 |
21 |
12 |
14 |
|
Ventricular premature beats |
3 |
21 |
10 |
19 |
|
Atrial fibrillation or flutter |
1 |
NR† |
12 |
NR† |
|
Multifocal atrial tachycardia |
0 |
NR† |
2 |
NR† |
|
Ventricular arrhythmias with hemodynamic instability |
7 |
14 |
40 |
0 |
|
Hypotension/ shock |
NR† |
21 |
NR† |
8 |
|
Neurologic | ||||
|
Nervousness |
NR† |
64 |
NR† |
21 |
|
Tremors |
38 |
29 |
16 |
14 |
|
Disorientation |
NR† |
7 |
NR† |
11 |
|
Seizures |
5 |
14 |
14 |
5 |
|
Death |
3 |
21 |
10 |
4 |
Other manifestations of theophylline toxicity include increases in serum calcium, creatine kinase, myoglobin and leukocyte count, decreases in serum phosphate and magnesium, acute myocardial infarction, and urinary retention in men with obstructive uropathy.
Seizures associated with serum theophylline concentrations >30 mcg/mL are often resistant to anticonvulsant therapy and may result in irreversible brain injury if not rapidly controlled. Death from theophylline toxicity is most often secondary to cardiorespiratory arrest and/or hypoxic encephalopathy following prolonged generalized seizures or intractable cardiac arrhythmias causing hemodynamic compromise.
Overdose Management
General Recommendations for Patients with Symptoms of Theophylline Overdose or Serum Theophylline Concentrations >30 mcg/mL while receiving intravenous theophylline.
- 1.
- Stop the theophylline infusion.
- 2.
- While simultaneously instituting treatment, contact a regional poison center to obtain updated information and advice on individualizing the recommendations that follow.
- 3.
- Institute supportive care, including establishment of intravenous access, maintenance of the airway, and electrocardiographic monitoring.
- 4.
- Treatment of seizures Because of the high morbidity and mortality associated with theophylline-induced seizures, treatment should be rapid and aggressive. Anticonvulsant therapy should be initiated with an intravenous benzodiazepine, e.g., diazepam, in increments of 0.1-0.2 mg/kg every 1-3 minutes until seizures are terminated. Repetitive seizures should be treated with a loading dose of phenobarbital (20 mg/kg infused over 30-60 minutes). Case reports of theophylline overdose in humans and animal studies suggest that phenytoin is ineffective in terminating theophylline-induced seizures. The doses of benzodiazepines and phenobarbital required to terminate theophylline-induced seizures are close to the doses that may cause severe respiratory depression or respiratory arrest; the clinician should therefore be prepared to provide assisted ventilation. Elderly patients and patients with COPD may be more susceptible to the respiratory depressant effects of anticonvulsants. Barbiturate-induced coma or administration of general anesthesia may be required to terminate repetitive seizures or status epilepticus. General anesthesia should be used with caution in patients with theophylline overdose because fluorinated volatile anesthetics may sensitize the myocardium to endogenous catecholamines released by theophylline. Enflurane appears less likely to be associated with this effect than halothane and may, therefore, be safer. Neuromuscular blocking agents alone should not be used to terminate seizures since they abolish the musculoskeletal manifestations without terminating seizure activity in the brain.
- 5.
- Anticipate Need for Anticonvulsants In patients with theophylline overdose who are at high risk for theophylline-induced seizures, e.g., patients with acute overdoses and serum theophylline concentrations >100 mcg/mL or chronic overdosage in patients >60 years of age with serum theophylline concentrations >30 mcg/mL, the need for anticonvulsant therapy should be anticipated. A benzodiazepine such as diazepam should be drawn into a syringe and kept at the patient’s bedside and medical personnel qualified to treat seizures should be immediately available. In selected patients at high risk for theophylline-induced seizures, consideration should be given to the administration of prophylactic anticonvulsant therapy. Situations where prophylactic anticonvulsant therapy should be considered in high risk patients include anticipated delays in instituting methods for extracorporeal removal of theophylline (e.g., transfer of a high risk patient from one health care facility to another for extracorporeal removal) and clinical circumstances that significantly interfere with efforts to enhance theophylline clearance (e.g., a neonate where dialysis may not be technically feasible or a patient with vomiting unresponsive to antiemetics who is unable to tolerate multiple-dose oral activated charcoal). In animal studies, prophylactic administration of phenobarbital, but not phenytoin, has been shown to delay the onset of theophylline-induced generalized seizures and to increase the dose of theophylline required to induce seizures (i.e., markedly increases the LD50). Although there are no controlled studies in humans, a loading dose of intravenous phenobarbital (20 mg/kg infused over 60 minutes) may delay or prevent life-threatening seizures in high risk patients while efforts to enhance theophylline clearance are continued. Phenobarbital may cause respiratory depression, particularly in elderly patients and patients with COPD.
- 6.
- Treatment of cardiac arrhythmias Sinus tachycardia and simple ventricular premature beats are not harbingers of life-threatening arrhythmias, they do not require treatment in the absence of hemodynamic compromise, and they resolve with declining serum theophylline concentrations. Other arrhythmias, especially those associated with hemodynamic compromise, should be treated with antiarrhythmic therapy appropriate for the type of arrhythmia.
- 7.
- Serum Theophylline Concentration Monitoring The serum theophylline concentration should be measured immediately upon presentation, 2-4 hours later, and then at sufficient intervals, e.g., every 4 hours, to guide treatment decisions and to assess the effectiveness of therapy. Serum theophylline concentrations may continue to increase after presentation of the patient for medical care as a result of continued absorption of theophylline from the gastrointestinal tract. Serial monitoring of serum theophylline concentrations should be continued until it is clear that the concentration is no longer rising and has returned to non-toxic levels.
- 8.
- General Monitoring Procedures Electrocardiographic monitoring should be initiated on presentation and continued until the serum theophylline level has returned to a non-toxic level. Serum electrolytes and glucose should be measured on presentation and at appropriate intervals indicated by clinical circumstances. Fluid and electrolyte abnormalities should be promptly corrected. Monitoring and treatment should be continued until the serum concentration decreases below 20 mcg/mL.
- 9.
- Enhance clearance of theophylline Multiple-dose oral activated charcoal (e.g., 0.5 mg/kg up to 20 g, every two hours) increases the clearance of theophylline at least twofold by adsorption of theophylline secreted into gastrointestinal fluids. Charcoal must be retained in, and pass through, the gastrointestinal tract to be effective; emesis should therefore be controlled by administration of appropriate antiemetics. Alternatively, the charcoal can be administered continuously through a nasogastric tube in conjunction with appropriate antiemetics. A single dose of sorbitol may be administered with the activated charcoal to promote stooling to facilitate clearance of the adsorbed theophylline from the gastrointestinal tract. Sorbitol alone does not enhance clearance of theophylline and should be dosed with caution to prevent excessive stooling which can result in severe fluid and electrolyte imbalances. Commercially available fixed combinations of liquid charcoal and sorbitol should be avoided in young children and after the first dose in adolescents and adults since they do not allow for individualization of charcoal and sorbitol dosing. In patients with intractable vomiting, extracorporeal methods of theophylline removal should be instituted (see OVERDOSAGE, Extracorporeal Removal).
Specific Recommendations:
Acute Overdose (e.g., excessive loading dose or excessive infusion rate for < 24 hours)
- 1.
-
Serum Concentration >20<30 mcg/mL
- A.
- Stop the theophylline infusion.
- B.
- Monitor the patient and obtain a serum theophylline concentration in 2-4 hours to insure that the concentration is decreasing.
- 2.
-
Serum Concentration >30<100 mcg/mL
- C.
- Stop the theophylline infusion.
- D.
- Administer multiple dose oral activated charcoal and measures to control emesis.
- E.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
- F.
- Institute extracorporeal removal if emesis, seizures, or cardiac arrhythmias cannot be adequately controlled (see OVERDOSAGE, Extracorporeal Removal).
- 3.
-
Serum Concentration >100 mcg/mL
- G.
- Stop the theophylline infusion.
- H.
- Consider prophylactic anticonvulsant therapy.
- I.
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- J.
- Consider extracorporeal removal, even if the patient has not experienced a seizure (see OVERDOSAGE, Extracorporeal Removal).
- K.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
Chronic Overdosage (e.g., excessive infusion rate for > than 24 hours)
- 1.
-
Serum Concentration >20<30 mcg/mL (with manifestations of theophylline toxicity)
- A.
- Stop the theophylline infusion.
- B.
- Monitor the patient and obtain a serum theophylline concentration in 2-4 hours to insure that the concentration is decreasing.
- 2.
-
Serum Concentration >30 mcg/mL in patients <60 years of age
- C.
- Stop the theophylline infusion.
- D.
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- E.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
- F.
- Institute extracorporeal removal if emesis, seizures, or cardiac arrhythmias cannot be adequately controlled (see OVERDOSAGE, Extracorporeal Removal).
- 3.
-
Serum Concentration >30 mcg/mL in patients ≥60 years of age.
- G.
- Stop the theophylline infusion.
- H.
- Consider prophylactic anticonvulsant therapy.
- I.
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- J.
- Consider extracorporeal removal even if the patient has not experienced a seizure (see OVERDOSAGE, Extracorporeal Removal).
- K.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
Extracorporeal Removal:
Increasing the rate of theophylline clearance by extracorporeal methods may rapidly decrease serum concentrations, but the risks of the procedure must be weighed against the potential benefit. Charcoal hemoperfusion is the most effective method of extracorporeal removal, increasing theophylline clearance up to six fold, but serious complications, including hypotension, hypocalcemia, platelet consumption and bleeding diatheses may occur. Hemodialysis is about as efficient as multiple-dose oral activated charcoal and has a lower risk of serious complications than charcoal hemoperfusion. Hemodialysis should be considered as an alternative when charcoal hemoperfusion is not feasible and multiple-dose oral charcoal is ineffective because of intractable emesis. Serum theophylline concentrations may rebound 5-10 mcg/mL after discontinuation of charcoal hemoperfusion or hemodialysis due to redistribution of theophylline from the tissue compartment. Peritoneal dialysis is ineffective for theophylline removal; exchange transfusions in neonates have been minimally effective.
DOSAGE AND ADMINISTRATION
General Considerations:
The steady-state peak serum theophylline concentration is a function of the infusion rate and the rate of theophylline clearance in the individual patient. Because of marked individual differences in the rate of theophylline clearance, the dose required to achieve a serum theophylline concentration in the 10-20 mcg/mL range varies fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance. For a given population there is no single theophylline dose that will provide both safe and effective serum concentrations for all patients. Administration of the median theophylline dose required to achieve a therapeutic serum theophylline concentration in a given population may result in either sub-therapeutic or potentially toxic serum theophylline concentrations in individual patients. The dose of theophylline must be individualized on the basis of peak serum theophylline concentration measurements in order to achieve a dose that will provide maximum potential benefit with minimal risk of adverse effects.
When theophylline is used as an acute bronchodilator, the goal of obtaining a therapeutic serum concentration is best accomplished with an intravenous loading dose. Because of rapid distribution into body fluids, the serum concentration (C) obtained from an initial loading dose (LD) is related primarily to the volume of distribution (V), the apparent space into which the drug diffuses:
C = LD/V
If a mean volume of distribution of about 0.5 L/kg is assumed (actual range is 0.3 to 0.7 L/kg), each mg/kg (ideal body weight) of theophylline administered as a loading dose over 30 minutes results in an average 2 mcg/mL increase in serum theophylline concentration. Therefore, in a patient who has received no theophylline in the previous 24 hours, a loading dose of intravenous theophylline of 4.6 mg/kg, calculated on the basis of ideal body weight and administered over 30 minutes, on average, will produce a maximum post-distribution serum concentration of 10 mcg/mL with a range of 6-16 mcg/mL. When a loading dose becomes necessary in the patient who has already received theophylline, estimation of the serum concentration based upon the history is unreliable, and an immediate serum level determination is indicated. The loading dose can then be determined as follows:
D = (Desired C - Measured C)(V)
where D is the loading dose, C is the serum theophylline concentration, and V is the volume of distribution. The mean volume of distribution can be assumed to be 0.5 L/kg and the desired serum concentration should be conservative (e.g., 10 mcg/mL) to allow for the variability in the volume of distribution. A loading dose should not be given before obtaining a serum theophylline concentration if the patient has received any theophylline in the previous 24 hours.
A serum concentration obtained 30 minutes after an intravenous loading dose, when distribution is complete, can be used to assess the need for and size of subsequent loading doses, if clinically indicated, and for guidance of continuing therapy. Once a serum concentration of 10 to 15 mcg/mL has been achieved with the use of a loading dose(s), a constant intravenous infusion is started. The rate of administration is based upon mean pharmacokinetic parameters for the population and calculated to achieve a target serum concentration of 10 mcg/mL (see Table VI). For example, in nonsmoking adults, initiation of a constant intravenous theophylline infusion of 0.4 mg/kg/hr at the completion of the loading dose, on average, will result in a steady-state concentration of 10 mcg/mL with a range of 7-26 mcg/mL. The mean and range of steady-state serum concentrations are similar when the average child (age 1 to 9 years) is given a loading dose of 4.6 mg/kg theophylline followed by a constant intravenous infusion of 0.8 mg/kg/hr. Since there is large interpatient variability in theophylline clearance, serum concentration will rise or fall when the patient’s clearance is significantly different from the mean population value used to calculate the initial infusion rate. Therefore, a second serum concentration should be obtained one expected half-life after starting the constant infusion (e.g., approximately 4 hours for children age 1 to 9 and 8 hours for nonsmoking adults; See Table II for the expected half-life in additional patient populations) to determine if the concentration is accumulating or declining from the post loading dose level. If the level is declining as a result of a higher than average clearance, an additional loading dose can be administered and/or the infusion rate increased. In contrast, if the second sample demonstrates a higher level, accumulation of the drug can be assumed, and the infusion rate should be decreased before the concentration exceeds 20 mcg/mL. An additional sample is obtained 12 to 24 hours later to determine if further adjustments are required and then at 24-hour intervals to adjust for changes, if they occur. This empiric method, based upon mean pharmacokinetic parameters, will prevent large fluctuations in serum concentration during the most critical period of the patient’s course.
In patients with cor pulmonale, cardiac decompensation, or liver dysfunction, or in those taking drugs that markedly reduce theophylline clearance (e.g., cimetidine), the initial theophylline infusion rate should not exceed 17 mg/hr unless serum concentrations can be monitored at 24-hour intervals. In these patients, 5 days may be required before steady-state is reached.
Theophylline distributes poorly into body fat, therefore, mg/kg dose should be calculated on the basis of ideal body weight.
Table VI contains initial theophylline infusion rates following an appropriate loading dose recommended for patients in various age groups and clinical circumstances. Table VII contains recommendations for final theophylline dosage adjustment based upon serum theophylline concentrations. Application of these general dosing recommendations to individual patients must take into account the unique clinical characteristics of each patient. In general, these recommendations should serve as the upper limit for dosage adjustments in order to decrease the risk of potentially serious adverse events associated with unexpected large increases in serum theophylline concentration.
|
||
|
Table VI. Initial theophylline infusion rates following an appropriate loading dose. |
||
|
Patient population |
Age | |
|
Neonates |
Postnatal age up to 24 days | |
|
Infants |
6-52 weeks old |
mg/kg/hr = (0.008) (age in weeks) + 0.21 |
|
Young children |
1-9 years |
0.8 |
|
Older children |
9-12 years |
0.7 |
|
Adolescents (cigarette or marijuana smokers) |
12-16 years |
0.7 |
|
Adolescents (nonsmokers) |
12-16 years |
0.5§ |
|
Adults (otherwise healthy nonsmokers) |
16-60 years |
0.4§ |
|
Elderly |
>60 years |
0.3¶ |
|
Cardiac decompensation, cor pulmonale, liver dysfunction, sepsis with multi-organ failure, or shock |
0.2¶ |
|
|
|
|
Table VII. Final dosage adjustment guided by serum theophylline concentration. |
|
|
Peak Serum Concentration |
Dosage Adjustment |
|
<9.9 mcg/mL |
If symptoms are not controlled and current dosage is tolerated, increase infusion rate about 25%. Recheck serum concentration after 12 hours in children and 24 hours in adults for further dosage adjustment. |
|
10 to 14.9 mcg/mL |
If symptoms are controlled and current dosage is tolerated, maintain infusion rate and recheck serum concentration at 24 hour intervals. * If symptoms are not controlled and current dosage is tolerated consider adding additional medication(s) to treatment regimen. |
|
15-19.9 mcg/mL |
Consider 10% decrease in infusion rate to provide greater margin of safety even if current dosage is tolerated.* |
|
20-24.9 mcg/mL |
Decrease infusion rate by 25% even if no adverse effects are present. Recheck serum concentration after 12 hours in children and 24 hours in adults to guide further dosage adjustment. |
|
25-30 mcg/mL |
Stop infusion for 12 hours in children and 24 hours in adults and decrease subsequent infusion rate at least 25% even if no adverse effects are present. Recheck serum concentration after 12 hours in children and 24 hours in adults to guide further dosage adjustment. If symptomatic, stop infusion and consider whether overdose treatment is indicated (see recommendations for chronic overdosage). |
|
>30 mcg/mL |
Stop the infusion and treat overdose as indicated (see recommendations for chronic overdosage). If theophylline is subsequently resumed, decrease infusion rate by at least 50% and recheck serum concentration after 12 hours in children and 24 hours in adults to guide further dosage adjustment. |
Many intravenous theophylline products are supplied as aminophylline where ethylenediamine is added to solubilize theophylline. Ethylenediamine is not required for solubility of premixed Theophylline and 5% Dextrose Injection. Each milligram of aminophylline dihydrate contains approximately 0.8 milligrams of theophylline anhydrous. Equivalent doses of premixed Theophylline and 5% Dextrose Injection can be determined by multiplying those doses specified as aminophylline dihydrate by 0.8.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
All injections in VIAFLEX Plus plastic containers are intended for intravenous administration using sterile equipment.
Because dosages of this drug are titrated to response, no additives should be made to Theophylline and 5% Dextrose Injections.
HOW SUPPLIED
Theophylline in 5% Dextrose Injection, USP in VIAFLEX Plus plastic container is available as follows:
|
Code |
Size (mL) |
NDC |
Product Name |
|
2B0896 |
50 |
0338-0445-41 |
200 mg Theophylline in |
|
2B0897 |
100 |
0338-0443-48 |
200 mg Theophylline in |
|
2B0887 |
100 |
0338-0445-48 |
400 mg Theophylline in |
|
2B0882 |
250 |
0338-0441-02 |
400 mg Theophylline in |
|
2B0883 |
500 |
0338-0439-03 |
400 mg Theophylline in |
|
2B0884 |
1000 |
0338-0437-04 |
400 mg Theophylline in |
|
2B0872 |
250 |
0338-0444-02 |
800 mg Theophylline in |
|
2B0873 |
500 |
0338-0441-03 |
800 mg Theophylline in |
|
2B0874 |
1000 |
0338-0439-04 |
800 mg Theophylline in |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
Caution: Federal (USA) law prohibits dispensing without prescription.
DIRECTIONS FOR USE OF VIAFLEX PLUS PLASTIC CONTAINER
Warning: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
To Open
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
Do not add supplementary medication.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
*Bar Code Position Only
071956843
07-19-56-843
Rev. March 2008
Baxter, Viaflex, and PL 146 are trademarks of Baxter International Inc.
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Container Label
500 mL Container Label
LOT EXP
2B0883 NDC 0338-0439-03
400 mg
THEOPHYLLINE
(0.8 mg/mL)
Theophylline in
5% Dextrose Injection USP
500 mL
EACH 100 mL CONTAINS 80 mg THEOPHYLLINE
ANHYDROUS USP 5 g DEXTROSE HYDROUS USP
pH 4.5 (3.5 TO 6.5) OSMOLARITY 257 mOsmol/L
(CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER DOSAGE AS DIRECTED BY A PHYSICIAN
ADMINISTER INTRAVENOUSLY USING A CALIBRATED INFUSION DEVICE SEE
DIRECTIONS CAUTIONS DO NOT ADD SUPPLEMENTARY MEDICATION MUST NOT
BE USED IN SERIES CONNECTIONS DO NOT ADMINISTER SIMULTANEOUSLY WITH
BLOOD DO NOT USE UNLESS SOLUTION IS CLEAR Rx ONLY
VIAFLEX PLUS CONTAINER
PL 146 PLASTIC
BAXTER PL 146 AND VIAFLEX ARE
TRADEMARKS OF BAXTER INTERNATIONAL INC
FOR PRODUCT INFORMATION
1-800-933-0303
Baxter
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA
Carton Label
500 mL Carton Label
Lot: x
Exp: x x
QTY: 18-500mL
Code: 2B0883
NDC: 0338-0439-03
THEOPHYLLINE 400 MG
AND 5% DEXTROSE INJECTION
(17) XX00 (10) x
(01) 50303380439039
LOT EXP
2B0873 NDC 0338-0441-03
800 mg
THEOPHYLLINE
(1.6 mg/mL)
Theophylline in
5% Dextrose Injection USP
500 mL
EACH 100 mL CONTAINS 160 mg THEOPHYLLINE
ANHYDROUS USP 5 g DEXTROSE HYDROUS USP
pH 4.5 (3.5 TO 6.5) OSMOLARITY 261 mOsmol/L
(CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER DOSAGE AS DIRECTED BY A PHYSICIAN
ADMINISTER INTRAVENOUSLY USING A CALIBRATED INFUSION DEVICE SEE
DIRECTIONS CAUTIONS DO NOT ADD SUPPLEMENTARY MEDICATION MUST NOT
BE USED IN SERIES CONNECTIONS DO NOT ADMINISTER SIMULTANEOUSLY WITH
BLOOD DO NOT USE UNLESS SOLUTION IS CLEAR Rx ONLY
VIAFLEX PLUS CONTAINER
PL 146 PLASTIC
BAXTER PL 146 AND VIAFLEX ARE
TRADEMARKS OF BAXTER INTERNATIONAL INC
FOR PRODUCT INFORMATION
1-800-933-0303
Baxter
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA
Lot: x
Exp: x x
QTY: 18-500mL
Code: 2B0873
NDC: 0338-0441-03
THEOPHYLLINE 800 MG
AND 5% DEXTROSE INJECTION
(17) XX00 (10) x
(01) 50303380441032
| THEOPHYLLINE IN DEXTROSE
theophylline anhydrous injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| THEOPHYLLINE IN DEXTROSE
theophylline anhydrous injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Corporation | 189326168 | MANUFACTURE(0338-0439, 0338-0441) | |