Label: SORE THROAT CHERRY- phenol spray
- NDC Code(s): 41250-164-06
- Packager: MEIJER, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat that occurs with high fever, headache, nausea, and vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
-
Directions
- adults and children 6 years of age and older
- apply to affected area (one spray)
- allow to remain in place for at least 15 seconds, then spit out
- use every 2 hours as directed by a doctor or dentist
- children under 12 years of age should be supervised in the use of this product
- children under 6 years of age, consult a doctor or dentist
- adults and children 6 years of age and older
- Other information
- Inactive ingredients
- Questions or comments?
-
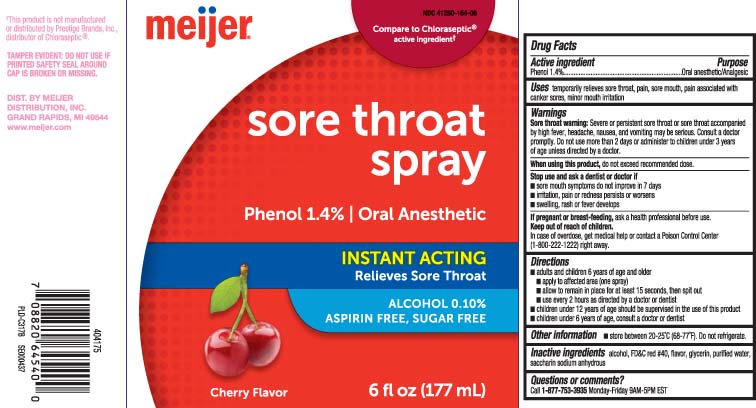
Principal Display Panel
Compare to Chloraseptic® active ingredient†
Sore Throat Spray
Phenol 1.4% | Oral Anesthetic
INSTANT ACTING
Relieves Sore Throat
ALCOHOL 0.10%
ASPIRIN FREE, SUGAR FREE
Cherry Flavor
FL OZ (mL)
†This product is not manufactured or distributed by Prestige Brands, Inc., distributor of Chloraseptic®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND CAP IS BROKEN OR MISSING.
DIST. BY MEIJER
DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
- Package Label
-
INGREDIENTS AND APPEARANCE
SORE THROAT CHERRY
phenol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-164 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 1.4 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color red (clear) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-164-06 177 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 05/01/2014 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/01/2014 12/31/2024 Labeler - MEIJER, INC. (006959555)