Label: CARBON DIOXIDE gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 48883-003-01, 48883-003-02, 48883-003-03, 48883-003-04, view more48883-003-05, 48883-003-06, 48883-003-07, 48883-003-08 - Packager: Encompass Medical & Specialty Gases, Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 3, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
CARBON DIOXIDE USP UN1013
NON-FLAMMABLE GAS 2 37048 (R 06/05)
Rx only. WARNING: Administration of Carbon Dioxide may be hazardous or contraindicated. For use only by or under supervision of a licensed practitioner who is experienced in the use and administration of Carbon Dioxide and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken. CAUTION: HIGH PRESSURE LIQUID AND GAS. CAN CAUSE RAPID SUFFOCATION. CAN INCREASE RESPIRATION AND HEART RATE. MAY CAUSE FROSTBITE. Avoid breathing gas. Store and use with adequate ventilation. Do not get liquid in eyes, on skin, and clothing. Cylinder temperatures should not exceed 52C (125F). Use equipment rated for cylinder pressure. Use a back flow preventive device in the piping. Close valve after each use and when empty. Use in accordance with the Material Safety Data Sheet (MSDS). FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Call a physician. IN CASE OF FROSTBITE, obtain medical treatment immediately. CAS: 124-38-9
DO NOT REMOVE THIS PRODUCT LABEL.
Cylinders must be refilled by:
Encompass Medical and Specialty Gases, LTD. 4646 Linden Road P.O. Box 5404 Rockford, IL 61109
CONTENTS: LTRS CU. FT.
-
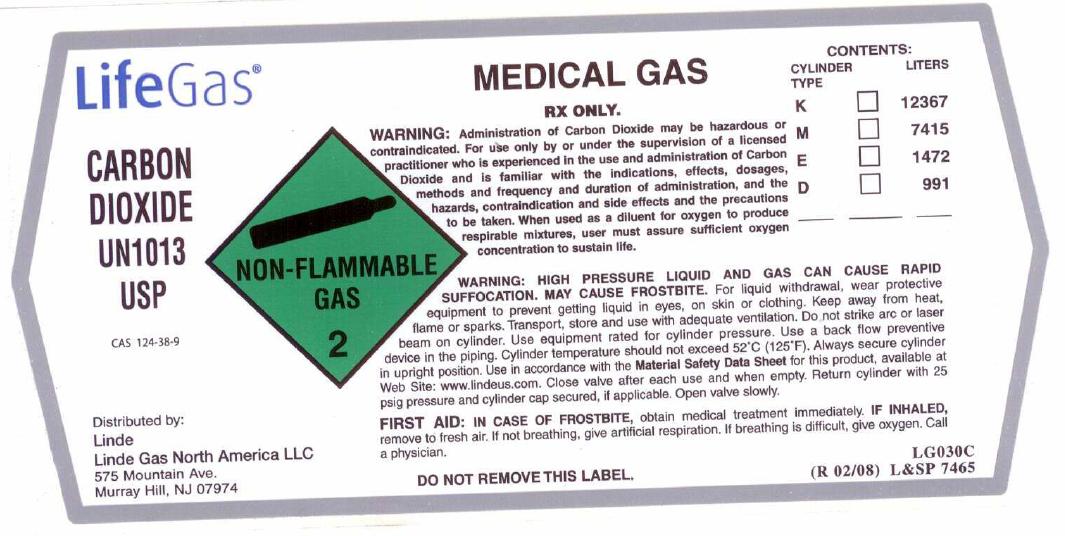
PRINCIPAL DISPLAY PANEL
LifeGas
CARBON DIOXIDE UN1013 USP
CAS: 124-38-9
Distributed by: Linde Linde Gas North America LLC 575 Mountain Ave. Murray Hill, NJ 07974
NON-FLAMMABLE GAS 2
MEDICAL GAS
Rx only. WARNING: Administration of Carbon Dioxide may be hazardous or contraindicated. For use only by or under supervision of a licensed practitioner who is experienced in the use and administration of Carbon Dioxide and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken. When used as a diluent for oxygen to produce respirable mixtures, user must assure sufficient oxygen concentration to sustain life.
WARNING: HIGH PRESSURE LIQUID AND GAS. CAN CAUSE RAPID SUFFOCATION. MAY CAUSE FROSTBITE. For liquid withdrawal, wear protective equipment to prevent getting liquid in eyes, on skin, or clothing. Keep away from heat, flame or sparks. Transport, store and use with adequate ventilation. Do not strike arc or laser beam on cylinder. Use equipment rated for cylinder pressure. Use a back flow preventive device in the piping. Cylinder temperatures should not exceed 52C (125F). Always secure cylinder in upright position. Use in accordance with the Material Safety Data Sheet for this product, available at Web Site: www.lindeus.com. Close valve after each use and when empty. Return cylinder with 25 psig pressure and cylinder cap secured, if applicable. Open valve slowly. FIRST AID: IN CASE OF FROSTBITE, obtain medical treatment immediately. IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Call a physician.
DO NOT REMOVE THIS LABEL.
LG030C (R 02/08) L and SP 7465
CONTENTS: CYLINDER TYPE K M E D
LITERS 12367 7415 1472 991
-
INGREDIENTS AND APPEARANCE
CARBON DIOXIDE
carbon dioxide gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:48883-003 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Carbon Dioxide (UNII: 142M471B3J) (Carbon Dioxide - UNII:142M471B3J) Carbon Dioxide 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48883-003-01 947 L in 1 CYLINDER 2 NDC:48883-003-02 991 L in 1 CYLINDER 3 NDC:48883-003-03 1472 L in 1 CYLINDER 4 NDC:48883-003-04 1597 L in 1 CYLINDER 5 NDC:48883-003-05 4961 L in 1 CYLINDER 6 NDC:48883-003-06 7415 L in 1 CYLINDER 7 NDC:48883-003-07 12367 L in 1 DEWAR 8 NDC:48883-003-08 12403 L in 1 DEWAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 01/02/2008 Labeler - Encompass Medical & Specialty Gases, Ltd. (963344143) Establishment Name Address ID/FEI Business Operations Encompass Medical & Specialty Gases, Ltd. 963344143 manufacture Establishment Name Address ID/FEI Business Operations Encompass Medical & Specialty Gases, Ltd. 963719286 manufacture Establishment Name Address ID/FEI Business Operations Encompass Medical & Specialty Gases, Ltd. 963719096 manufacture Establishment Name Address ID/FEI Business Operations Encompass Medical & Specialty Gases, Ltd. 963718361 manufacture