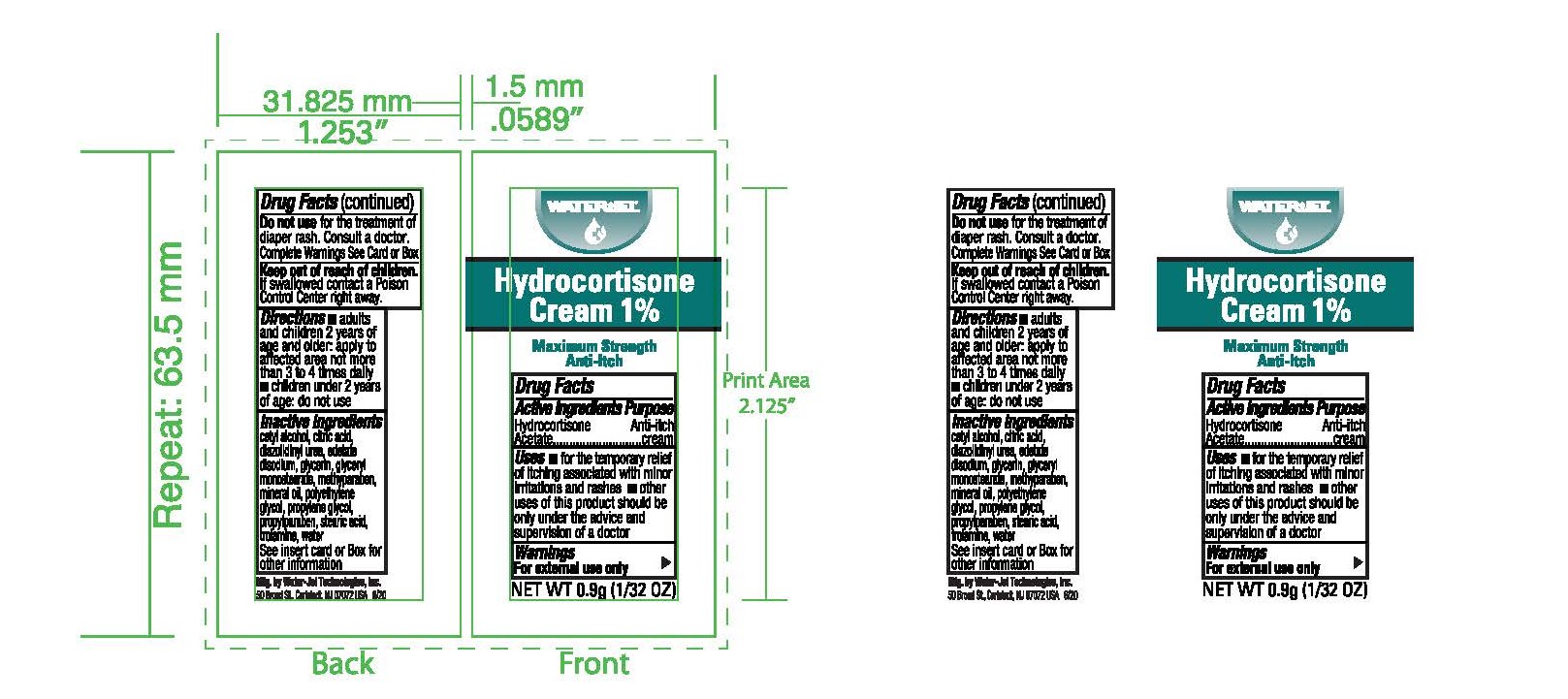
Label: HYDROCORTISONE- anti-itch cream ointment
-
NDC Code(s):
59898-800-01,
59898-800-02,
59898-800-03,
59898-800-32, view more59898-800-36
- Packager: Water-Jel Technologies
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions ?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
anti-itch cream ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59898-800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) LIGHT MINERAL OIL (UNII: N6K5787QVP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-800-03 25 in 1 BOX, UNIT-DOSE 04/30/2010 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:59898-800-02 144 in 1 BOX, UNIT-DOSE 04/30/2010 2 0.9 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:59898-800-01 1728 in 1 CARTON 04/30/2010 3 0.9 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:59898-800-36 0.9 g in 1 PACKET; Type 0: Not a Combination Product 04/30/2010 5 NDC:59898-800-32 28 g in 1 TUBE; Type 0: Not a Combination Product 04/30/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/30/2010 Labeler - Water-Jel Technologies (155522589) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(59898-800)