STOOL SOFTENER- docusate sodium capsule, liquid filled
International Laboratories, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
International Laboratories, Inc. Docusate Sodium 100 mg Drug Facts
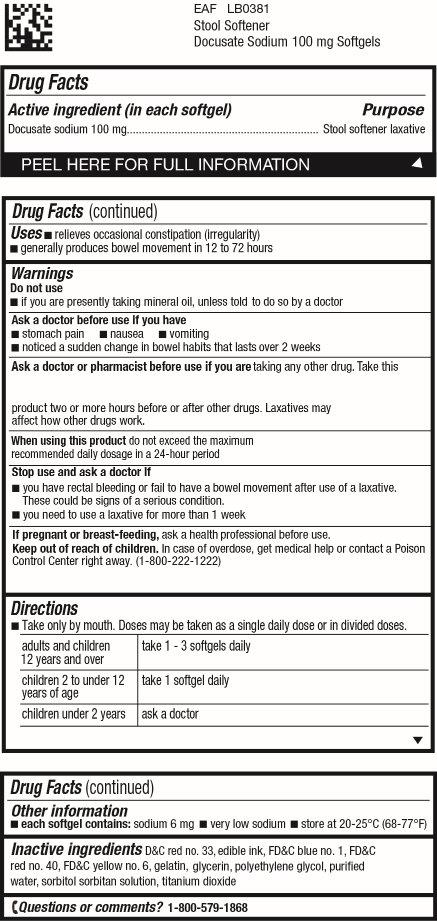
Uses
• relieves occasional constipation (irregularity)
• generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
• stomach pain
• nausea
• vomiting
• noticed a sudden change in bowel habits that lasts over 2 weeks
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
Directions
• Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1-3 softgels daily |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
| STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - International Laboratories, LLC (023569924) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| L. Perrigo Company | 006013346 | MANUFACTURE(54458-853) , RELABEL(54458-853) , REPACK(54458-853) | |
Revised: 12/2017
Document Id: 7001677a-14b6-4d51-a91a-b0021472d082
Set id: 3d2211af-9742-49e8-9016-51902e52bde4
Version: 5
Effective Time: 20171205
International Laboratories, LLC