SYNTHAMIN- leucine, isoleucine, lysine, valine, phenylalanine, histidine, threonine, methionine, tryptophan, alanine, arginine, glycine, proline, serine, tyrosine injection, solution
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
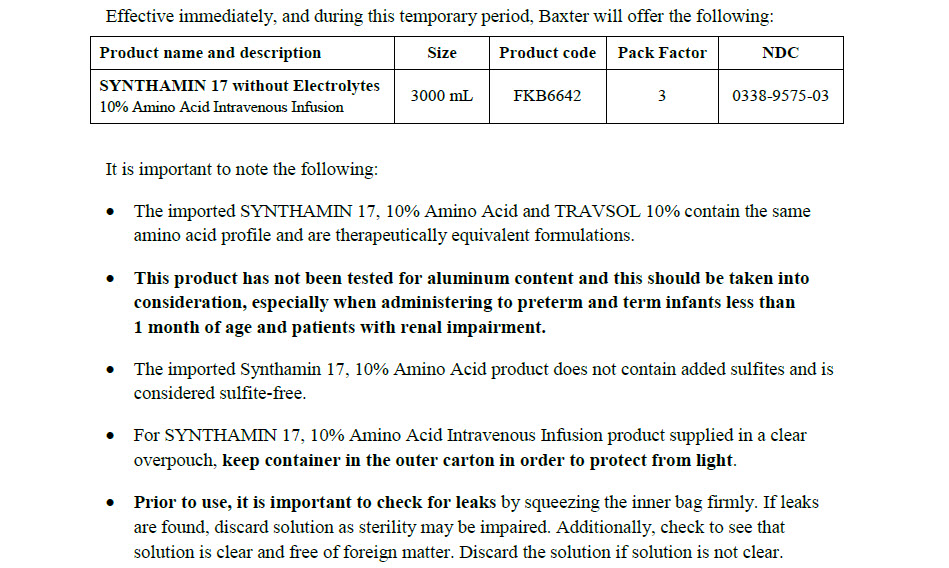
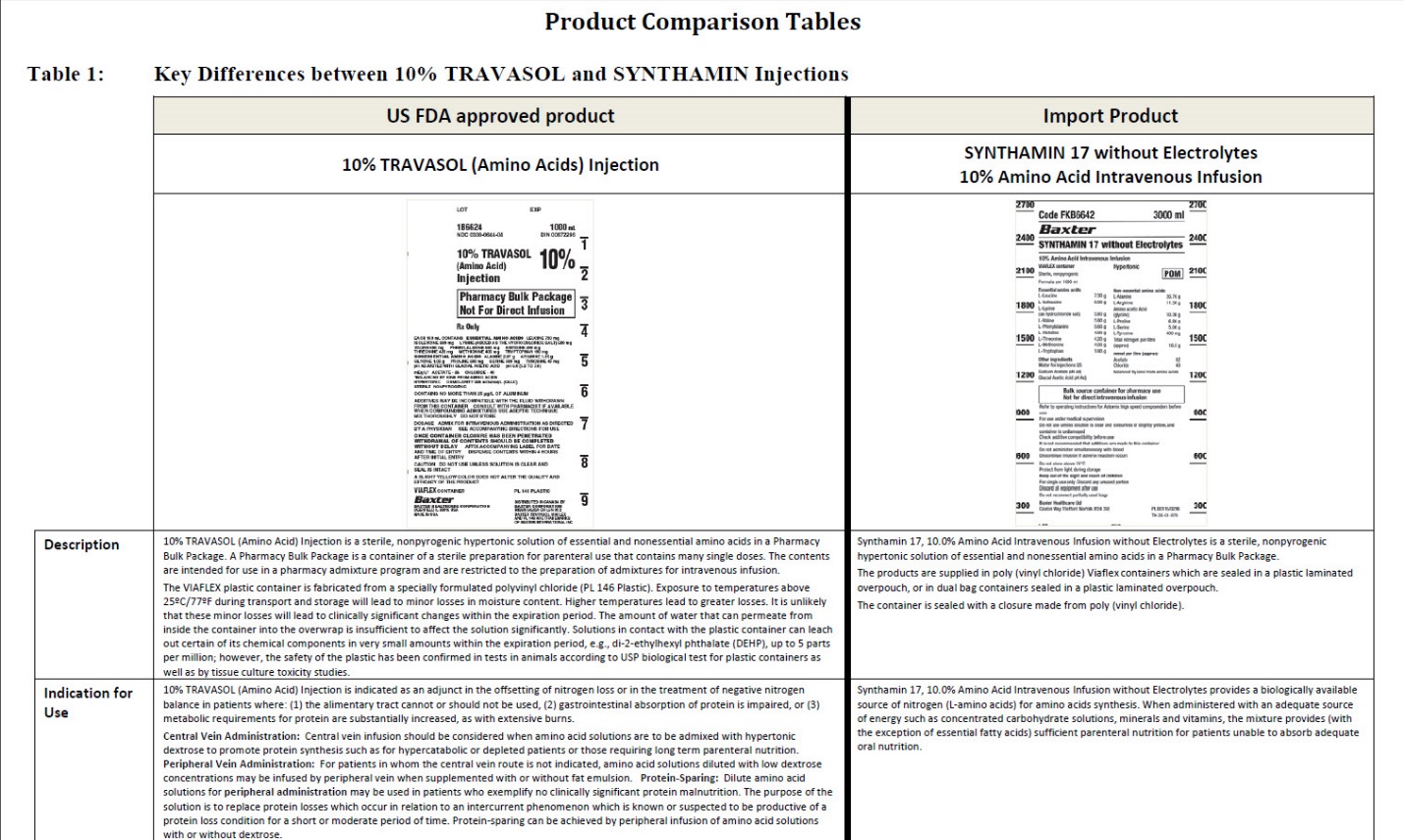
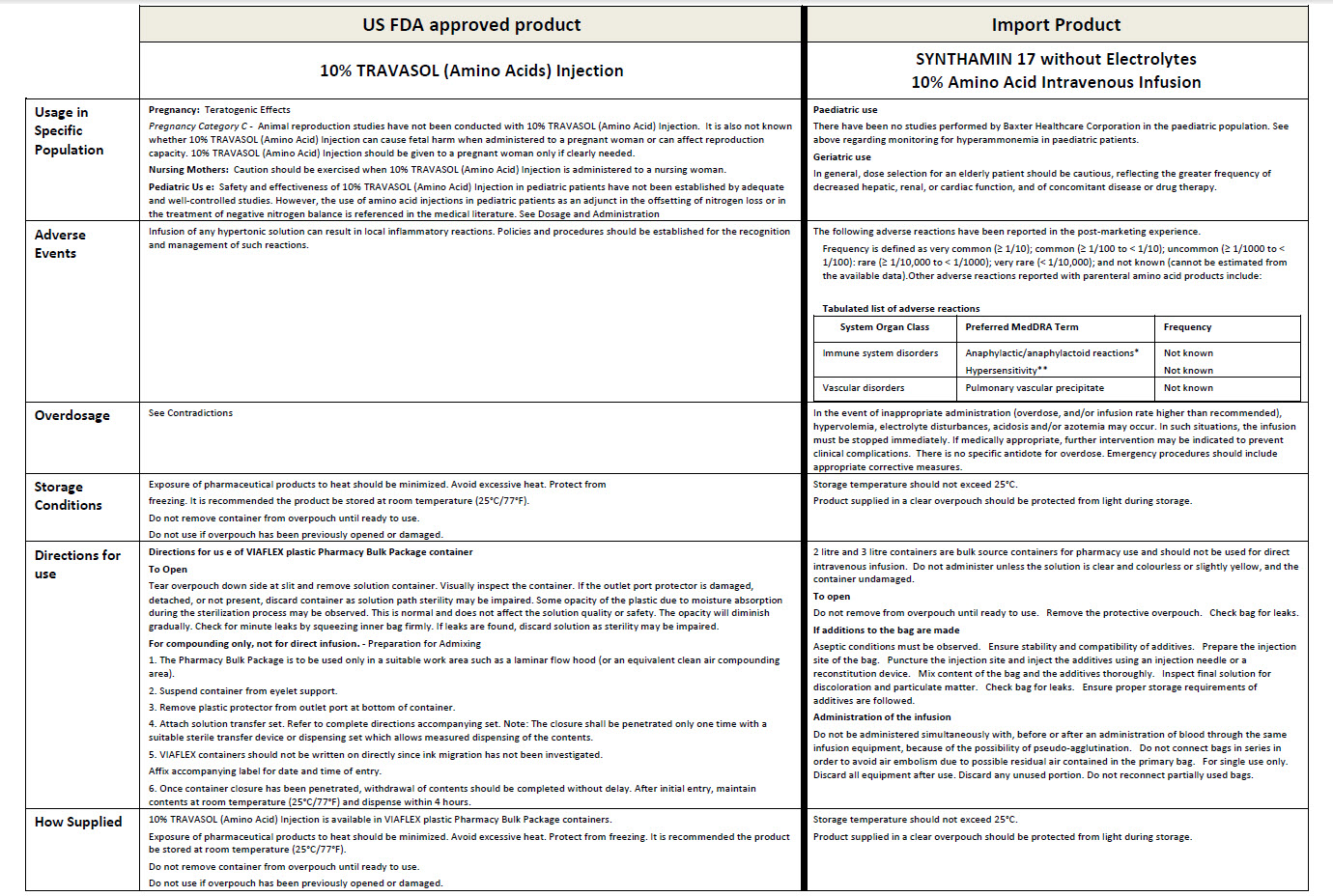
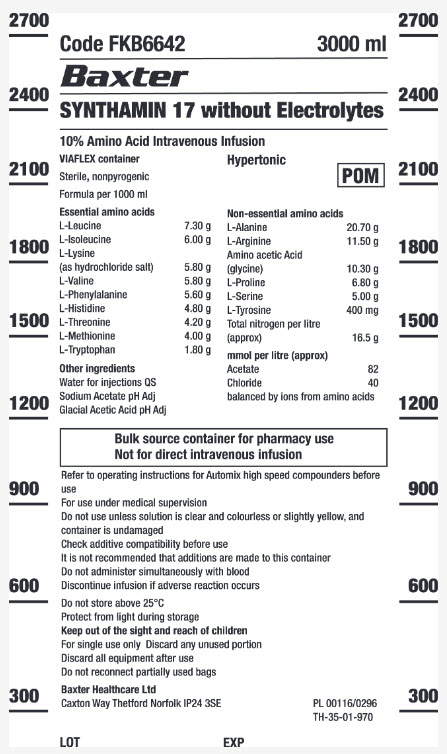
SYNTHAMIN 17 without Electrolytes
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Code FKB6642
3000 ml
Baxter Logo
SYNTHAMIN 17 without Electrolytes
10% Amino Acid Intravenous Infusion
VIAFLEX container
Hypertonic
POM symbol
Sterile, nonpyrogenic
Formula per 1000 ml
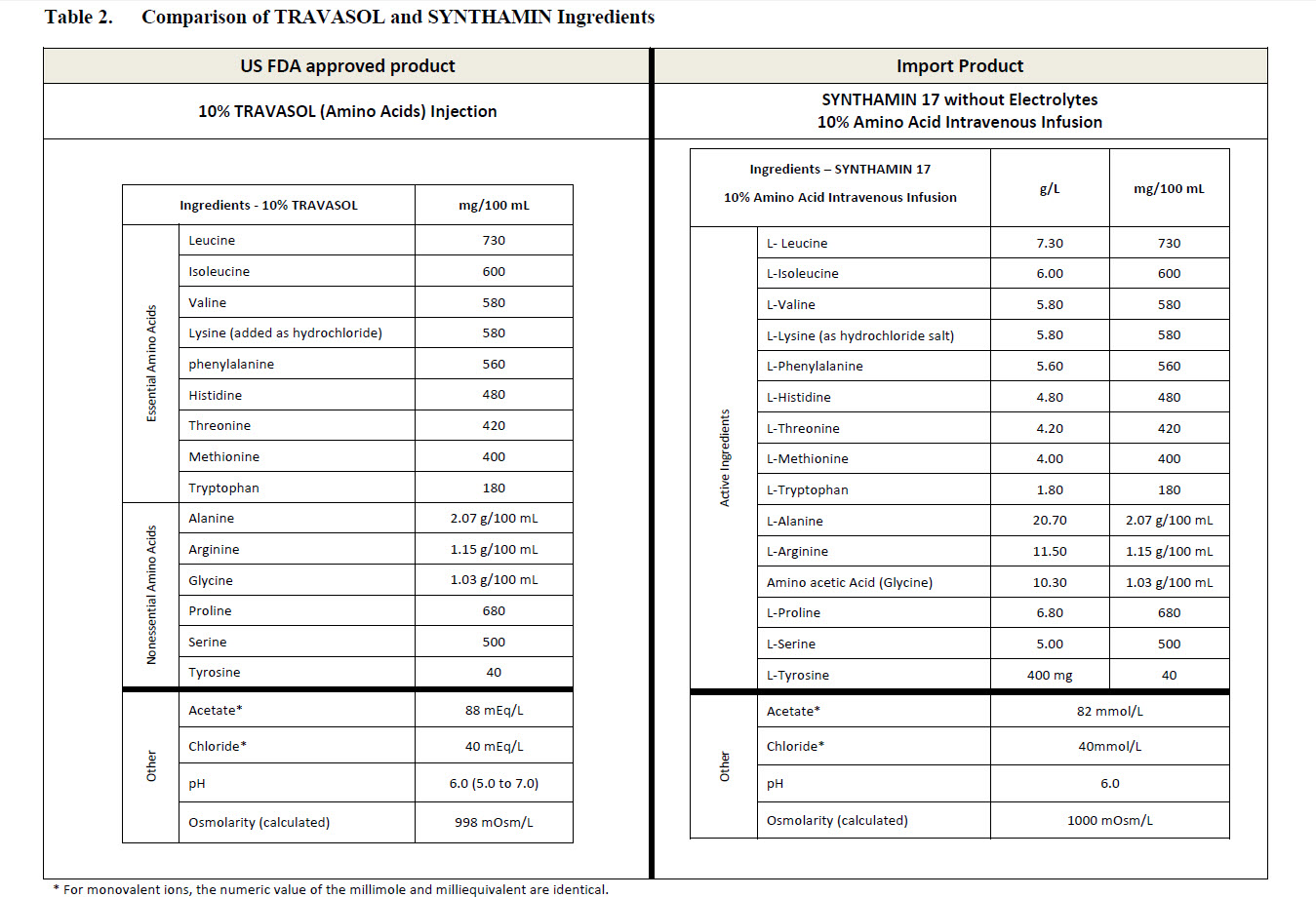
Essential amino acids
L-Leucine 7.30 g
L-Isoleucine 6.00 g
L-Lycine (as hydrochloride salt) 5.80 g
L-Valine 5.80 g
L-Phenylatanine 5.80 g
L-Histidine 4.80 g
L-Threonine 4.20 g
L-Methionine 4.00 g
L-Trypotophan 1.80 g
Other Ingredients
Water for injections QS
Sodium Acetate pH Adj
Glacial Acetic Acid pH Adj
Non-essential amino acids
L-Alanine 20.70 g
L-Arginine 11.50 g
Amino acetic Acid (glycine) 10.30 g
L-Proline 6.80 g
L-Serine 5.00 g
L-Tyrosine 400 mg
Total nitrogen per litre (approx) 18.5 g
mmol per litre (approx.)
Acetate 82
Chloride 40
balanced by ions from amino acids
Bulk source container for pharmacy use
Not for direct intravenous infusion
Refer to operating instructions for Automix high speed compounders before
use
For use under medical supervision
Do not use unless solution is clear and colourless or slightly yellow, and
container is undamaged
Check additive compatibility before use
It is not recommended that additions are made to this container
Do not administer simultaneously with blood
Discontinue infusion if adverse reaction occurs
Do not store above 25°C
Protect from light during storage
Keep out of the sight and reach of children
For single use only Discard any usused portion
Discard all equipment after use
Do not reconnect partially used bags
Baxter Healthcare Ltd
Caxton Way Thetford Norfolk IP24 3SE
PL 00116/0296
TH-35-01-970
LOT
EXP
2700
2400
2100
1800
1500
1200
900
600
300
| SYNTHAMIN
leucine, isoleucine, lysine, valine, phenylalanine, histidine, threonine, methionine, tryptophan, alanine, arginine, glycine, proline, serine, tyrosine injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Ltd | 221478644 | ANALYSIS(0338-9575) , MANUFACTURE(0338-9575) , LABEL(0338-9575) , PACK(0338-9575) , STERILIZE(0338-9575) | |