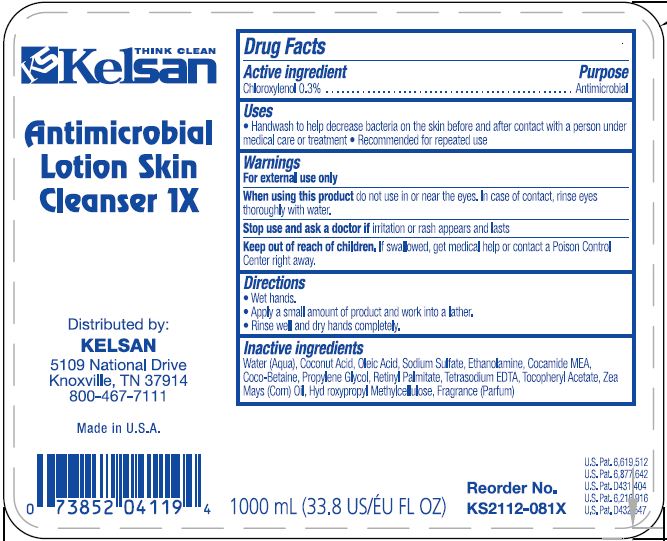
KELSAN ANTIMICROBIAL LTION SKIN CLEANSER- chloroxylenol liquid
Kel-San, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KELSAN Antimicrobial Lotion Skin Cleanser
Warnings
For external use only
Directions
• Wet hands
• Apply a small amount of product and work into a lather
• Rinse well and dry hands completely
| KELSAN ANTIMICROBIAL LTION SKIN CLEANSER
chloroxylenol liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Kel-San, Inc. (108349937) |
Revised: 12/2020
Document Id: 35d13785-c0fe-4b00-99de-ffedd5b7b9c2
Set id: 3cfeae6b-0b6a-4894-b8c0-85df830989cd
Version: 2
Effective Time: 20201222
Kel-San, Inc.