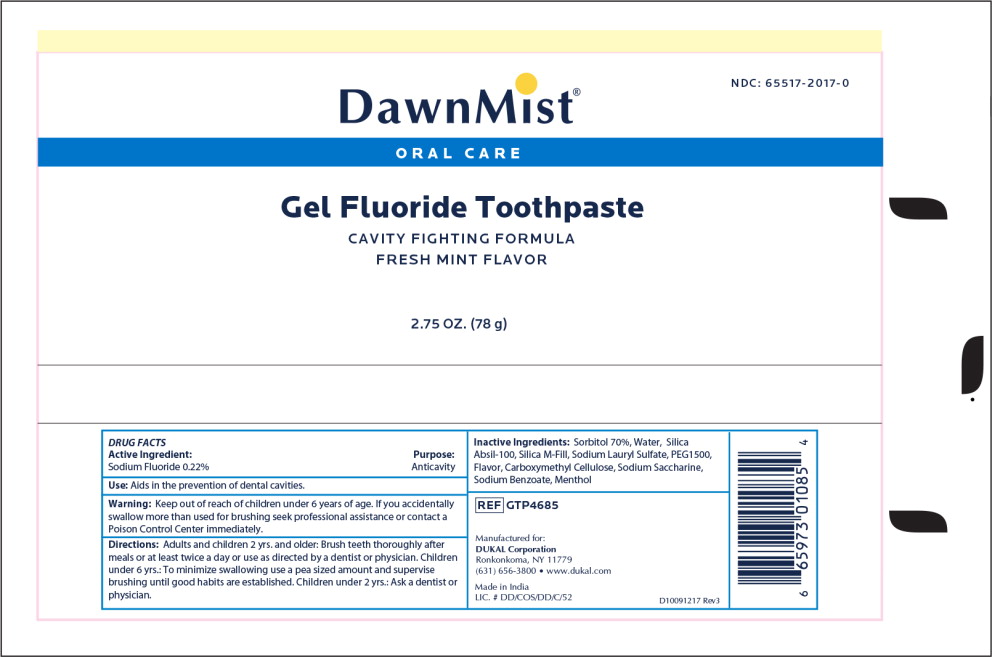
Label: DAWNMIST FLUORIDE- sodium fluoride paste
- NDC Code(s): 65517-2017-0, 65517-2017-1, 65517-2017-2, 65517-2017-3
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DawnMist Gel Fluoride Toothpaste
- Active Ingredient
- Purpose
- Use
- Warning:
- Keep out of reach of children under 6 years of age
-
Directions
Adults and children 2 years and older: Brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist or physician.
Children under 6 years: To minimize swallowing, use a peas sized amount and supervisor brushing until good habits are established.
Children under 2 years: Ask a dentist or physician
- Inactive Ingredient
- Principal Display Panel - DawnMist Gel Fluoride Toothpaste 2.75 oz Tube Label
-
INGREDIENTS AND APPEARANCE
DAWNMIST FLUORIDE
sodium fluoride pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-2017 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.22 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) SODIUM BENZOATE (UNII: OJ245FE5EU) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-2017-0 78 g in 1 TUBE; Type 0: Not a Combination Product 07/14/2017 2 NDC:65517-2017-1 17 g in 1 TUBE; Type 0: Not a Combination Product 07/14/2017 3 NDC:65517-2017-2 24 g in 1 TUBE; Type 0: Not a Combination Product 07/14/2017 4 NDC:65517-2017-3 43 g in 1 TUBE; Type 0: Not a Combination Product 07/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/17/2014 Labeler - Dukal LLC (791014871)