EXECLEAR
C- codeine phosphate and guaifenesin liquid
Larken Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ExeClear-C
DESCRIPTION
ExeClear-C Syrup is a clear, colorless liquid with a fruit gum flavor for oral administration.
Each teaspoon (5 ml) of ExeClear-C Syrup contains:
Codeine* Phosphate USP …………………. 10 mg
(*Warning: May be habit Forming)
Guaifenesin USP ………………………………. 200 mg
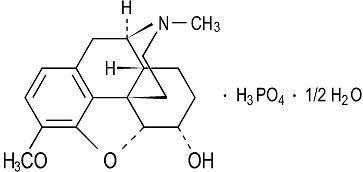
Codeine phosphate has the chemical name 7, 8-Didehydro-4,5α-epoxy-3-methoxy-17-methylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate. Its molecular formula is C 18H 21NO 3H 3PO 4 • ½H 2O with a molecular weight of 406.37. It occurs as fine, white, needle-shaped crystals, or white, crystalline powder. It is freely soluble in water at 25°C; slightly soluble in ethanol. Following absorption codeine is metabolized by the liver and metabolic products are excreted in the urine. Its structure is as follows:
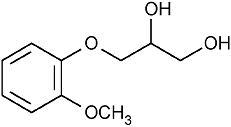
Guaifenesin has the chemical name 3-(2-methoxyphenoxy)-1,2-propanediol. Its molecular formula is C 10H 14O 4, with a molecular weight of 198.21. It is a white or slightly gray crystalline substance with a slightly bitter aromatic taste. One gram dissolves in 20 mL water at 25°C; freely soluble in ethanol. Guaifenesin is readily absorbed from the GI tract and is rapidly metabolized and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite is beta-(2-methoxyphenoxy) lactic acid. Its structure is as follows:
INACTIVE INGREDIENTS
Propylene glycol, Glycerin, Sodium benzoate, Citric acid, Saccharin sodium, Magnasweet, Flavor, Sorbitol, and Purified water.
CLINICAL PHARMACOLOGY
ExeClear-C Syrup combines the expectorant, guaifenesin and the cough suppressant, codeine. Guaifenesin is an expectorant the action of which promotes or facilitates the removal of secretions from the respiratory tract. By increasing sputum volume and making sputum less viscous, guaifenesin facilitates expectoration of retained secretions. Codeine is a centrally acting antitussive agent.
INDICATIONS AND USAGE
Temporarily relieves cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants. Calms the cough control center and relieves coughing. Helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus, drain bronchial tubes, and make coughs more productive.
PRECAUTIONS
General
Ultra-rapid Metabolizers of Codeine
Some individuals may be ultra-rapid metabolizers due to a specific CYP2D6*2x2 genotype. These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regiments, individuals who are ultra-rapid metabolizers may experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1-10% in Caucasians, 3% in African Americans, and 16-28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups. When physicians prescribe codeine-containing drugs, they should choose the lowest effective dose for the shortest period of time and should inform their patients about these risks and the signs of morphine overdose. (See
PRECAUTIONS-Nursing Mothers.)
Information for Patients
Patients should be warned about engaging in activities requiring mental alertness, such as driving a car or operating dangerous machinery. Caution patients that some people have a variation in a liver enzyme and change codeine into morphine more rapidly and completely than other people. These people are ultra-rapid metabolizers and are more likely to have higher-than normal levels of morphine in their blood after taking codeine which can result in overdose symptoms such as extreme sleepiness, confusion, or shallow breathing. In most cases, it is unknown if someone is an ultra-rapid codeine metabolizer. Nursing mothers taking codeine can also have higher morphine levels in their breast milk if they are ultra-rapid metabolizers. These higher levels of morphine in breast milk may lead to life-threatening or fatal side effects in nursing babies. Instruct nursing mothers to watch for signs of morphine toxicity in their infants including increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to the baby’s doctor immediately if they notice these signs and, if they cannot reach the doctor right away, to take the baby to an emergency room or call 911 (or local emergency services).
Drug Interactions
The use of codeine may result in additive CNS depressant effects when co administered with alcohol, antihistamines, psychotropics, or other drugs which produce CNS depression.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies of ExeClear-C Syrup to assess the long-term carcinogenic and mutagenic potential or the effect on fertility in animals or humans have not been performed.
Pregnancy
Teratogenic Effects
Pregnancy-Category C:Teratogenic Effects: Animal reproduction studies have not been conducted. Safe use in pregnancy has not been established relative to possible adverse effects on fetal development. Therefore, this product should not be used in pregnant patients unless, in the judgment of the physician, the potential benefits outweigh possible hazards.
Nonteratogenic Effects
Nonteratogenic Effects: Dependence has been reported in newborns whose mothers took opiates regularly during pregnancy. Withdrawal signs include irritability, excessive crying, tremors, hyper-reflexia, fever, vomiting, and diarrhea. Signs usually appear during the first few days of life.
Labor and Delivery
Labor and Delivery: Narcotic analgesics cross the placental barrier. The closer to delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Narcotic analgesics should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required.
Nursing Mothers
Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose-dependent. Despite the common use of codeine products to manage postpartum pain, reports of adverse events in infants are rare. However, some women are ultra-rapid metabolizers of codeine. These women achieve higher-than-expected serum levels of codeine’s active metabolite, morphine, leading to higher-than-expected levels of morphine in breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, maternal use of codeine can potentially lead to serious adverse reactions, including death, in nursing infants. The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1-10% in Caucasians, 3% in African Americans, and 16-28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups. The risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and baby. Caution should be exercised when codeine is administered to a nursing woman. If a codeine containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Mothers using codeine should be informed about when to seek immediate medical care and how to identify the signs and symptoms of neonatal toxicity, such as drowsiness or sedation, difficulty breastfeeding, breathing difficulties, and decreased tone, in their baby. Nursing mothers who are ultra-rapid metabolizers may also experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. Prescribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine
during breastfeeding. (See
PRECAUTIONS - General - Ultra-rapid Metabolizers of Codeine.)
DRUG & OR LABORATORY TEST INTERACTIONS
Laboratory Test Interactions: Guaifenesin or its metabolites may cause color interference with the vanillylmandelic acid (VMA) test for catechols. It may also falsely elevate the level of urinary 5-hydroxyindoleacetic acid (5-HIAA) in certain serotonin metabolite chemical tests because of color interference.
ADVERSE REACTIONS
Guaifenesin is well tolerated and has a wide margin of safety. Nausea and vomiting are the side effects that occur most commonly. Other reported adverse reactions have included dizziness, headache, and rash (including urticaria). Codeine: Nausea, vomiting, constipation, drowsiness, and miosis have been reported. Higher doses may induce euphoria, light headedness, dizziness, drowsiness, and depression of respiration. Pruritus and skin rashes have been rare.
DRUG ABUSE AND DEPENDENCE
Controlled Substance - Schedule V Dependence - Codeine may be habit forming.
OVERDOSAGE
If overdosage occurs, treat symptomatically. Gastric emptying (emesis and/or gastric lavage) is recommended. Severe intoxication with codeine may result in dyspnea, vertigo, double vision, delusions, hallucinations, speech disturbances, excitement, restlessness, delirium, constricted pupils, respiratory depression (slow and shallow breathing), Cheyne-Stokes respiration, circulatory collapse, stupor, and coma. Treatment of overdosage consists primarily of support of vital functions, especially management of codeine-induced respiratory depression. The narcotic antagonist naloxone is a specific antidote for respiratory depression which may result from overdose or unusual sensitivity from narcotics.
DOSAGE AND ADMINISTRATION
Adults and children 12 years of age and older: 2 teaspoonfuls every 4 to 6 hours, not to exceed 12 teaspoonfuls. in 24 hours.
Children 6 years to under 12 years of age: 1 teaspoonful every 4 to 6 hours, not to exceed 6 teaspoonfuls in 24 hours.
Children 5 years to under 6 years of age (avg. 18kg (40 lbs)): 1⁄2 teaspoonful every 4 to 6 hours, not to exceed 2 teaspoonfuls in 24 hours.
Children 3 years to under 5 years of age (avg. 14kg (30 lbs) to 16kg (35 lbs)): 1⁄4 teaspoonful every 4 to 6 hours, not to exceed 1 teaspoonful in 24 hours.
Not recommended for children under the age of 3 unless as directed by a physician. Parents should be instructed to obtain and use a dispensing device (such as a dropper calibrated for age or weight) to administer the drug to a child, to use extreme care in measuring dosage, and not to exceed recommended daily dosage.
CODEINE IS NOT RECOMMENDED FOR USE IN CHILDREN UNDER 2 YEARS OF AGE. Children under 2 years of age may be more susceptible to the respiratory depressant effects of codeine, including respiratory arrest, coma, and death.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN AND TO SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY IN CASE OF ACCIDENTAL OVERDOSE.
HOW SUPPLIED
Guaifenesin 200 mg and codeine phosphate 10 mg per 5 ml of clear, colorless liquid in bottles of 16 oz (473 ml) (NDC 68047-222-16).
Storage and Handling
Storage - Store at controlled room temperature - 15°-30°C (59°-86°F). Protect from light. Keep bottle tightly closed.
Distributed by:
Larken Laboratories, Inc.
Madison, MS 39110
Questions or Comments: 888-527-5522
Manufactured by:
Deltex Pharmaceuticals, Inc.
Rosenburg, TX 77471
Rev. 09/08
| EXECLEAR
C
codeine phosphate / guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Larken Laboratories, Inc. (149484540) |
| Registrant - Larken Laboratories, Inc. (149484540) |
