NOTUSS-NXD- codeine phosphate, pseudoephedrine hcl, chlorcyclizine hcl liquid
SJ PHARMACEUTICALS, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Notuss-NXD
DESCRIPTION:
Each 5 mL (one teaspoonful) for oral administration contains:
Codeine Phosphate .................................................. 10 mg
(WARNING: May be habit forming)
Pseudoephedrine HCl .............................................. 30 mg
Chlorcyclizine HCl ............................................... 9.375 mg
Active Ingredients Purpose
(in each 5 mL teaspoonful)
Codeine Phosphate 10 mg .................................. Antitussive
Pseudoephedrine Hydrochloride 30 mg ........... Decongestant
Chlorcyclizine Hydrochloride 9.375 mg .............. Antihistamine
USES:
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose - sneezing - itching of the nose or throat - itchy, watery eyes - cough due to minor throat and bronchial irritation - nasal congestion - reduces swelling of the nasal passages
WARNINGS:
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm
- a chronic pulmonary disease or shortness of breath, or children who are taking other drugs
- heart disease
- high blood pressure
- thyroid disease
- diabetes
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using this product
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- may cause or aggravate constipation
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occurs
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
- new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 12 years of age and older:
2 teaspoonfuls (10 mL) every 6 to 8 hours, not to exceed 8 teaspoonfuls in a 24 hour period.
Children under 12 years of age:
Consult a doctor
Do not exceed recommended dosage.
PRODUCT PACKAGING:
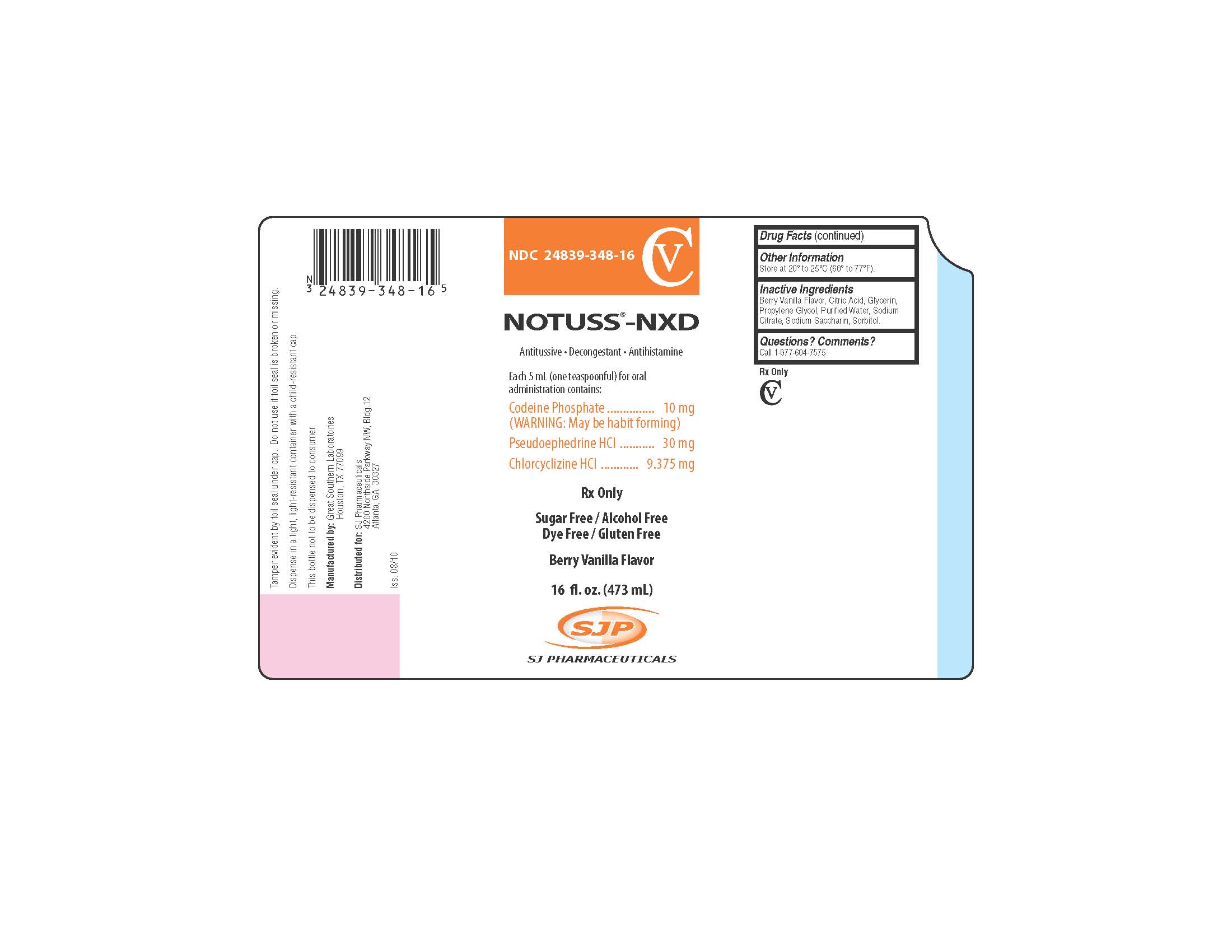
NOTUSS®-NXD
Antitussive - Decongestant - Antihistamine
Each 5 mL (one teaspoonful) for oral administration contains:
Codeine Phosphate .................................................. 10 mg
(WARNING: May be habit forming)
Pseudoephedrine HCl .............................................. 30 mg
Chlorcyclizine HCl ............................................... 9.375 mg
Rx Only
Sugar Free/Alcohol Free
Dye Free/Gluten Free
Berry Vanilla Flavor
16 fl. oz. (473 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This bottle not to be dispensed to consumer.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Distributed for:
SJ Pharmaceuticals
4200 Northside Parkway NW, Bldg. 12
Atlanta, GA 30327
Iss. 08/10

| NOTUSS-NXD
codeine phosphate, pseudoephedrine hcl, chlorcyclizine hcl liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - SJ PHARMACEUTICALS, LLC (845662720) |
| Registrant - GREAT SOUTHERN LABORATORIES (056139553) |