Label: LORATADINE tablet
- NDC Code(s): 0378-8880-10
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
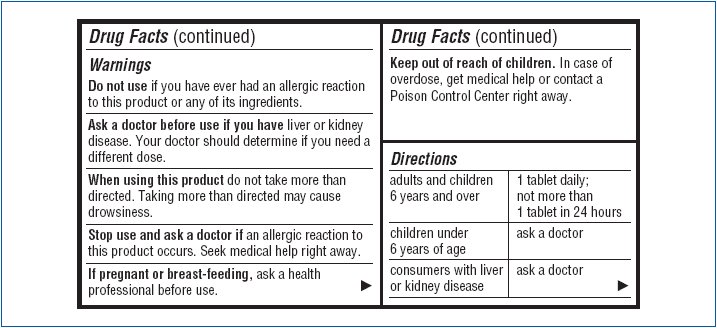
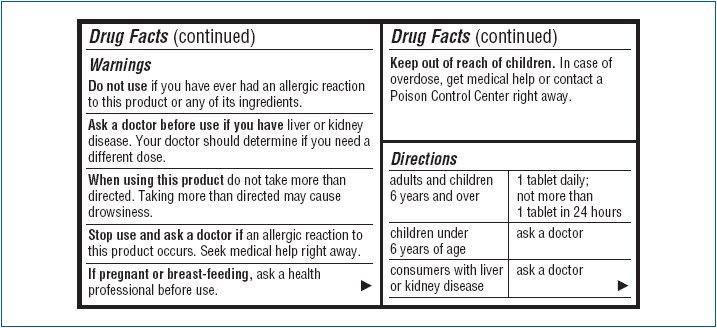
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
PRODUCT PACKAGING
NDC 0378-8880-10
Original Prescription Strength
Non-Drowsy*
Loratadine
Tablets USP, 10 mgAntihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose
*When taken as directed. See Drug Facts Panel.
RMX8880C1
100 Tablets
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0378-8880 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 6mm Flavor Imprint Code G;L;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-8880-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076154 06/01/2011 Labeler - Mylan Pharmaceuticals Inc. (059295980)