EPIVIR- lamivudine tablet
REMEDYREPACK INC.
----------
BOXED WARNING
LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, INCLUDING FATAL CASES, HAVE BEEN REPORTED WITH THE USE OF NUCLEOSIDE ANALOGUES ALONE OR IN COMBINATION, INCLUDING LAMIVUDINE AND OTHER ANTIRETROVIRALS (SEE WARNINGS).
HUMAN IMMUNODEFICIENCY VIRUS (HIV) COUNSELING AND TESTING SHOULD BE OFFERED TO ALL PATIENTS BEFORE BEGINNING EPIVIR-HBV AND PERIODICALLY DURING TREATMENT (SEE WARNINGS), BECAUSE EPIVIR-HBV TABLETS AND ORAL SOLUTION CONTAIN A LOWER DOSE OF THE SAME ACTIVE INGREDIENT (LAMIVUDINE) AS EPIVIRTABLETS AND ORAL SOLUTION USED TO TREAT HIV INFECTION. IF TREATMENT WITH EPIVIR-HBV IS PRESCRIBED FOR CHRONIC HEPATITIS B FOR A PATIENT WITH UNRECOGNIZED OR UNTREATED HIV INFECTION, RAPID EMERGENCE OF HIV RESISTANCE IS LIKELY BECAUSE OF SUBTHERAPEUTIC DOSE AND INAPPROPRIATE MONOTHERAPY.
SEVERE ACUTE EXACERBATIONS OF HEPATITIS B HAVE BEEN REPORTED IN PATIENTS WHO HAVE DISCONTINUED ANTI-HEPATITIS B THERAPY (INCLUDING EPIVIR-HBV). HEPATIC FUNCTION SHOULD BE MONITORED CLOSELY WITH BOTH CLINICAL AND LABORATORY FOLLOW-UP FOR AT LEAST SEVERAL MONTHS IN PATIENTS WHO DISCONTINUE ANTI-HEPATITIS B THERAPY. IF APPROPRIATE, INITIATION OF ANTI-HEPATITIS B THERAPY MAY BE WARRANTED (SEE WARNINGS).
DESCRIPTION
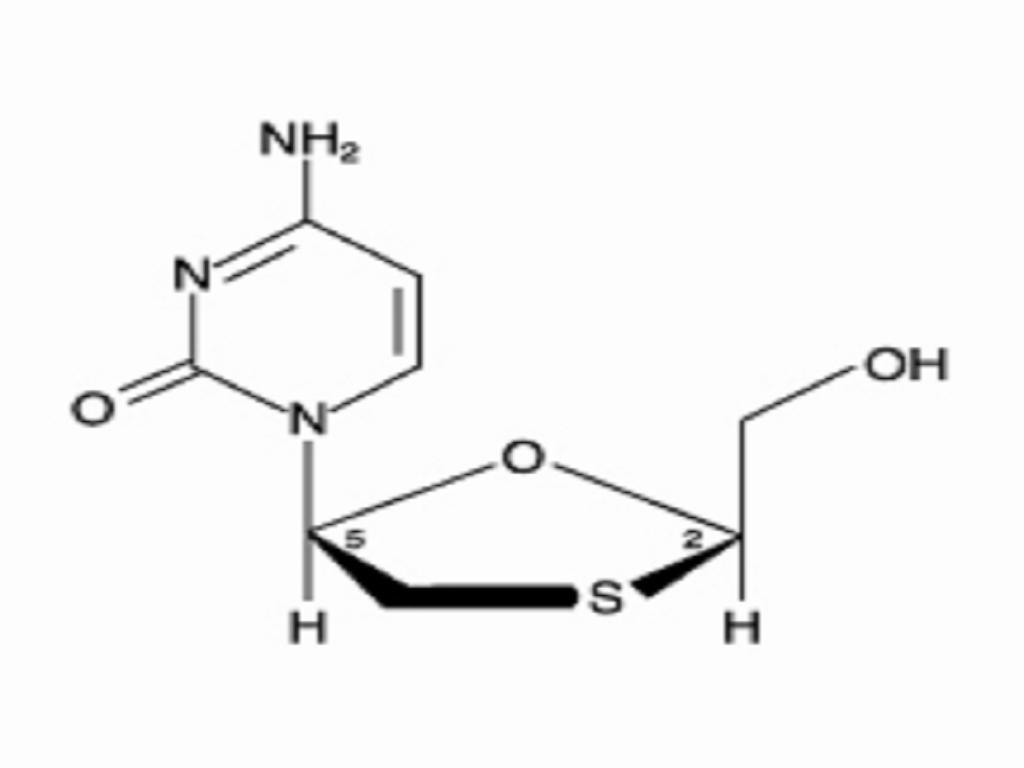
EPIVIR-HBV is a brand name for lamivudine, a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Lamivudine was initially developed for the treatment of HIV infection as EPIVIR. Please see the complete prescribing information for EPIVIR Tablets and Oral Solution for additional information. The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)23It has a molecular formula of C8H11N3O3S and a molecular weight of 229.3. It has the following structural formula:
Lamivudine is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20
EPIVIR-HBV Tablets are for oral administration. Each tablet contains 100 mg of lamivudine and the inactive ingredients hypromellose, macrogol 400, magnesium stearate, microcrystalline cellulose, polysorbate 80, red iron oxide, sodium starch glycolate, titanium dioxide, and yellow iron oxide.
EPIVIR-HBV Oral Solution is for oral administration. One milliliter (1 mL) of EPIVIR-HBV Oral Solution contains 5 mg of lamivudine (5 mg/mL) in an aqueous solution and the inactive ingredients artificial strawberry and banana flavors, citric acid (anhydrous), methylparaben, propylene glycol, propylparaben, sodium citrate (dihydrate), and sucrose (200 mg).
MICROBIOLOGY
Mechanism of Action:
Lamivudine is a synthetic nucleoside analogue. Intracellularly, lamivudine is phosphorylated to its active 5metabolite, lamivudine triphosphate, 3TC-TP. Incorporation of the monophosphate form into viral DNA by HBV reverse transcriptase results in DNA chain termination. 3TC-TP also inhibits the RNA- and DNA-dependent DNA polymerase activities of HIV-1 reverse transcriptase (RT). 3TC-TP is a weak inhibitor of mammalianandpolymerases.
Antiviral Activity:
Activity of lamivudine against HBV in cell culture was assessed in HBV DNA-transfected 2.2.15 cells, HB611 cells, and infected human primary hepatocytes. EC50 values (the concentration of drug needed to reduce the level of extracellular HBV DNA by 50%) varied from 0.01(2.3 ng/mL) to 5.6(1.3 mcg/mL) depending upon the duration of exposure of cells to lamivudine, the cell model system, and the protocol used. See the EPIVIR package insert for information regarding activity of lamivudine against HIV.
Resistance:
Lamivudine-resistant isolates were identified in patients with virologic breakthrough, defined when using solution hybridization assay as the detection of HBV DNA in serum on 2 or more occasions after failing to detect HBV DNA on 2 or more occasions and defined when using PCR assay as a >1 log10 (10-fold) increase in serum HBV DNA from nadir during treatment in a patient who had an initial virologic response.
Lamivudine-resistant HBV isolates develop M204V/I substitutions in the YMDD motif of the catalytic domain of the viral reverse transcriptase. M204V/I substitutions are frequently accompanied by other substitutions (V173L, L180M) which enhance the level of lamivudine resistance or act as compensatory mutations improving replication efficiency. Other substitutions detected in lamivudine-resistant HBV isolates include L80I and A181T.
In 4 controlled clinical trials in adults with HBeAg-positive chronic hepatitis B virus infection (CHB), YMDD-mutant HBV was detected in 81 of 335 patients receiving lamivudine 100 mg once daily for 52 weeks. The prevalence of YMDD substitutions was less than 10% in each of these trials for patients studied at 24 weeks and increased to an average of 24% (range in 4 studies: 16% to 32%) at 52 weeks. In limited data from a long-term follow-up trial in patients who continued 100 mg/day lamivudine after one of these studies, YMDD substitutions further increased from 18% (10 of 57) at 1 year to 41% (20 of 49), 53% (27 of 51), and 69% (31 of 45) after 2, 3, and 4 years of treatment, respectively. Over the 5-year treatment period, the proportion of patients who developed YMDD-mutant HBV at any time was 69% (40 of 58).
In a controlled trial in pediatric patients, YMDD-mutant HBV was detected in 31 of 166 (19%) patients receiving lamivudine for 52 weeks. For a subgroup who remained on lamivudine therapy in a follow-up study, YMDD mutations increased from 24% (29 of 121) at 12 months to 59% (68 of 115) at 24 months and 64% (66 of 103) at 36 months of lamivudine treatment.
In a controlled study, treatment-naive patients with HBeAg-positive CHB were treated with lamivudine or lamivudine plus adefovir dipivoxil combination therapy. Following 104 weeks of therapy, YMDD-mutant HBV was detected in 7 of 40 (18%) patients receiving combination therapy compared with 15 of 35 (43%) patients receiving lamivudine-only therapy. In another controlled study, combination therapy was evaluated in adult patients with HBeAg-positive CHB who had YMDD-mutant HBV and diminished clinical and virologic response to lamivudine. Following 52 weeks of lamivudine plus adefovir dipivoxil combination therapy (n = 46) or lamivudine-only therapy (n = 49), YMDD-mutant HBV was detected less frequently in patients receiving combination therapy, 62% vs 96%.
A published study suggested that the rates of lamivudine resistance in patients treated for HBeAg-negative CHB appear to be more variable (0% to 27% at 1 year and 10% to 56% at 2 years).
Cross-Resistance:
HBV: HBV containing lamivudine resistance-associated substitutions (rtL180M, rtM204I, rtM204V, rtL180M + rtM204V, rtV173L + rtL180M + rtM204V) retain susceptibility to adefovir dipivoxil but have reduced susceptibility to entecavir (30 fold) and telbivudine (>100 fold). The lamivudine resistance-associated substitution rtA181T results in diminished response to adefovir and telbivudine. Similarly, HBV with entecavir resistance-associated substitutions (I169T/M250V and T184G/S202I) have >1,000-fold reductions in susceptibility to lamivudine.
HIV: In studies of HIV-1-infected patients who received lamivudine monotherapy or combination therapy with lamivudine plus zidovudine for at least 12 weeks, HIV-1 isolates with reduced susceptibility in cell culture to lamivudine were detected in most patients (see WARNINGS).
CONTRAINDICATIONS
EPIVIR-HBV Tablets and EPIVIR-HBV Oral Solution are contraindicated in patients with previously demonstrated clinically significant hypersensitivity to any of the components of the products.
WARNINGS
Lactic Acidosis/Severe Hepatomegaly With Steatosis:
Important Differences Between Lamivudine-Containing Products, HIV Testing, and Risk of Emergence of Resistant HIV:
EPIVIR-HBV Tablets and Oral Solution contain a lower dose of the same active ingredient (lamivudine) as EPIVIR Tablets and Oral Solution, COMBIVIR(lamivudine/zidovudine) Tablets, EPZICOM(abacavir sulfate and lamivudine) Tablets, and TRIZIVIR(abacavir, lamivudine, and zidovudine) Tablets used to treat HIV infection. The formulation and dosage of lamivudine in EPIVIR-HBV are not appropriate for patients dually infected with HBV and HIV. If a decision is made to administer lamivudine to such patients, the higher dosage indicated for HIV therapy should be used as part of an appropriate combination regimen, and the prescribing information for EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR as well as for EPIVIR-HBV should be consulted. HIV counseling and testing should be offered to all patients before beginning EPIVIR-HBV and periodically during treatment because of the risk of rapid emergence of resistant HIV and limitation of treatment options if EPIVIR-HBV is prescribed to treat chronic hepatitis B in a patient who has unrecognized or untreated HIV infection or acquires HIV infection during treatment.
;
Posttreatment Exacerbations of Hepatitis:
Pancreatitis:
Pancreatitis has been reported in patients receiving lamivudine, particularly in HIV-infected pediatric patients with prior nucleoside exposure.
PRECAUTIONS
General:
Patients should be assessed before beginning treatment with EPIVIR-HBV by a physician experienced in the management of chronic hepatitis B.
Emergence of Resistance-Associated HBV Mutations:
In controlled clinical trials, YMDD-mutant HBV were detected in patients with on-lamivudine re-appearance of HBV DNA after an initial decline below the solution-hybridization assay limit (see MICROBIOLOGY: Drug Resistance). These mutations can be detected by a research assay and have been associated with reduced susceptibility to lamivudine in vitro. Lamivudine-treated patients (adult and pediatric) with YMDD-mutant HBV at 52 weeks showed diminished treatment responses in comparison to lamivudine-treated patients without evidence of YMDD mutations, including lower rates of HBeAg seroconversion and HBeAg loss (no greater than placebo recipients), more frequent return of positive HBV DNA by solution-hybridization or branched-chain DNA assay, and more frequent ALT elevations. In the controlled trials, when patients developed YMDD-mutant HBV, they had a rise in HBV DNA and ALT from their own previous on-treatment levels. Progression of hepatitis B, including death, has been reported in some patients with YMDD-mutant HBV, including patients from the liver transplant setting and from other clinical trials. In clinical practice, monitoring of ALT and HBV DNA levels during lamivudine treatment may aid in treatment decisions if emergence of viral mutants is suspected.
Limitations of Populations Studied:
Assessing Patients During Treatment:
Patients should be monitored regularly during treatment by a physician experienced in the management of chronic hepatitis B. The safety and effectiveness of treatment with EPIVIR-HBV beyond 1 year have not been established. During treatment, combinations of such events such as return of persistently elevated ALT, increasing levels of HBV DNA over time after an initial decline below assay limit, progression of clinical signs or symptoms of hepatic disease, and/or worsening of hepatic necroinflammatory findings may be considered as potentially reflecting loss of therapeutic response. Such observations should be taken into consideration when determining the advisability of continuing therapy with EPIVIR-HBV.
The optimal duration of treatment, the durability of HBeAg seroconversions occurring during treatment, and the relationship between treatment response and long-term outcomes such as hepatocellular carcinoma or decompensated cirrhosis are not known.
Patients With Impaired Renal Function:
Reduction of the dosage of EPIVIR-HBV is recommended for patients with impaired renal function (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
INFORMATION FOR PATIENTS
A Patient Package Insert (PPI) for EPIVIR-HBV is available for patient information.
Patients should remain under the care of a physician while taking EPIVIR-HBV. They should discuss any new symptoms or concurrent medications with their physician.
Patients should be advised that EPIVIR-HBV is not a cure for hepatitis B, that the long-term treatment benefits of EPIVIR-HBV are unknown at this time, and, in particular, that the relationship of initial treatment response to outcomes such as hepatocellular carcinoma and decompensated cirrhosis is unknown. Patients should be informed that deterioration of liver disease has occurred in some cases when treatment was discontinued. Patients should be advised to discuss any changes in regimen with their physician.
Patients should be informed that emergence of resistant hepatitis B virus and worsening of disease can occur during treatment, and they should promptly report any new symptoms to their physician.
Patients should be counseled on the importance of testing for HIV to avoid inappropriate therapy and development of resistant HIV, and HIV counseling and testing should be offered before starting EPIVIR-HBV and periodically during therapy. Patients should be advised that EPIVIR-HBV Tablets and EPIVIR-HBV Oral Solution contain a lower dose of the same active ingredient (lamivudine) as EPIVIR Tablets, EPIVIR Oral Solution, COMBIVIR Tablets, EPZICOM Tablets, and TRIZIVIR Tablets. EPIVIR-HBV should not be taken concurrently with EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR (see WARNINGS). Patients infected with both HBV and HIV who are planning to change their HIV treatment regimen to a regimen that does not include EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR should discuss continued therapy for hepatitis B with their physician.
Patients should be advised that treatment with EPIVIR-HBV has not been shown to reduce the risk of transmission of HBV to others through sexual contact or blood contamination (see Pregnancy section).
Diabetic patients should be advised that each 20-mL dose of EPIVIR-HBV Oral Solution contains 4 grams of sucrose.
DRUG INTERACTIONS
Lamivudine is predominantly eliminated in the urine by active organic cationic secretion. The possibility of interactions with other drugs administered concurrently should be considered, particularly when their main route of elimination is active renal secretion via the organic cationic transport system (e.g., trimethoprim).
TMP 160 mg/SMX 800 mg once daily has been shown to increase lamivudine exposure (AUC) by 44% (see CLINICAL PHARMACOLOGY). No change in dose of either drug is recommended. There is no information regarding the effect on lamivudine pharmacokinetics of higher doses of TMP/SMX such as those used to treat Pneumocystis carinii pneumonia. No data are available regarding interactions with other drugs that have renal clearance mechanisms similar to that of lamivudine.
Lamivudine and zalcitabine may inhibit the intracellular phosphorylation of one another. Therefore, use of lamivudine in combination with zalcitabine is not recommended.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Lamivudine long-term carcinogenicity studies in mice and rats showed no evidence of carcinogenic potential at exposures up to 34 times (mice) and 200 times (rats) those observed in humans at the recommended therapeutic dose for chronic hepatitis B. Lamivudine was not active in a microbial mutagenicity screen or an in vitro cell transformation assay, but showed weak in vitro mutagenic activity in a cytogenetic assay using cultured human lymphocytes and in the mouse lymphoma assay. However, lamivudine showed no evidence of in vivo genotoxic activity in the rat at oral doses of up to 2,000 mg/kg producing plasma levels of 60 to 70 times those in humans at the recommended dose for chronic hepatitis B. In a study of reproductive performance, lamivudine administered to rats at doses up to 4,000 mg/kg/day, producing plasma levels 80 to 120 times those in humans, revealed no evidence of impaired fertility and no effect on the survival, growth, and development to weaning of the offspring.
PREGNANCY
Pregnancy Category C. Reproduction studies have been performed in rats and rabbits at orally administered doses up to 4,000 mg/kg/day and 1,000 mg/kg/day, respectively, producing plasma levels up to approximately 60 times that for the adult HBV dose. No evidence of teratogenicity due to lamivudine was observed. Evidence of early embryolethality was seen in the rabbit at exposure levels similar to those observed in humans, but there was no indication of this effect in the rat at exposures up to 60 times that in humans. Studies in pregnant rats and rabbits showed that lamivudine is transferred to the fetus through the placenta. There are no adequate and well-controlled studies in pregnant women. Because animal reproductive toxicity studies are not always predictive of human response, lamivudine should be used during pregnancy only if the potential benefits outweigh the risks.
Lamivudine has not been shown to affect the transmission of HBV from mother to infant, and appropriate infant immunizations should be used to prevent neonatal acquisition of HBV.
Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to lamivudine, a Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
NURSING MOTHERS
A study in lactating rats administered 45 mg/kg of lamivudine showed that lamivudine concentrations in milk were slightly greater than those in plasma. Lamivudine is also excreted in human milk. Samples of breast milk obtained from 20 mothers receiving lamivudine monotherapy (300 mg twice daily) or combination therapy (150 mg lamivudine twice daily and 300 mg zidovudine twice daily) had measurable concentrations of lamivudine.
Because of the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving lamivudine.
PEDIATRIC USE
HBV: Safety and efficacy of lamivudine for treatment of chronic hepatitis B in children have been studied in pediatric patients from 2 to 17 years of age in a controlled clinical trial (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, and DOSAGE AND ADMINISTRATION).
Safety and efficacy in pediatric patients <2 years of age have not been established.
HIV: See the complete prescribing information for EPIVIR Tablets and Oral Solution for additional information on pharmacokinetics of lamivudine in HIV-infected children.
GERIATRIC USE
Clinical studies of EPIVIR-HBV did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In particular, because lamivudine is substantially excreted by the kidney and elderly patients are more likely to have decreased renal function, renal function should be monitored and dosage adjustments should be made accordingly (see PRECAUTIONS: Patients with Impaired Renal Function and DOSAGE AND ADMINISTRATION).<
ADVERSE REACTIONS
Several serious adverse events reported with lamivudine (lactic acidosis and severe hepatomegaly with steatosis, posttreatment exacerbations of hepatitis B, pancreatitis, and emergence of viral mutants associated with reduced drug susceptibility and diminished treatment response) are also described in WARNINGS and PRECAUTIONS.
Clinical Trials In Chronic Hepatitis B:
Adults: Selected clinical adverse events observed with afrequency during therapy with EPIVIR-HBV compared with placebo are listed in Table 5. Frequencies of specified laboratory abnormalities during therapy with EPIVIR-HBV compared with placebo are listed in Table 6.
Table 5. Selected Clinical Adverse Events (Frequency) in 3 Placebo-Controlled Clinical Trials in Adults During Treatmenta (Studies 1-3)
EPIVIR-HBVPlaceboAdverse Event(n = 332)(n = 200)Non-site SpecificMalaise and fatigue24%28%Fever or chills7%9%Ear, Nose, and ThroatEar, nose, and throat infections25%21%Sore throat13%8%GastrointestinalNausea and vomiting15%17%Abdominal discomfort and pain16%17%Diarrhea14%12%MusculoskeletalMyalgia14%17%Arthralgia7%5%NeurologicalHeadache21%21%SkinSkin rashes5%5%a Includes patients treated for 52 to 68 weeks.
Table 6. Frequencies of Specified Laboratory Abnormalities in 3 Placebo-Controlled Trials in Adults During Treatmenta (Studies 1-3)
Test Patients With Abnormality/Patients With Observations (Abnormal Level) EPIVIR-HBV Placebo ALT >3 x baselineb 37/331 (11%) 26/199 (13%) Albumin <2.5 g/dL 0/331 (0%) 2/199 (1%) Amylase >3 x baseline 2/259 (<1%) 4/167 (2%) Serum Lipasex ULNc 19/189 (10%) 9/127 (7%) CPKx baseline 31/329 (9%) 9/198 (5%) Neutrophils <750/mm3 0/331 (0%) 1/199 (<1%) Platelets <50,000/mm3 10/272 (4%) 5/168 (3%)
a Includes patients treated for 52 to 68 weeks.
b See Table 7 for posttreatment ALT values.
c Includes observations during and after treatment in the 2 placebo-controlled trials that collected this information.
ULN = Upper limit of normal.
In patients followed for up to 16 weeks after discontinuation of treatment, posttreatment ALT elevations were observed more frequently in patients who had received EPIVIR-HBV than in patients who had received placebo. A comparison of ALT elevations between Weeks 52 and 68 in patients who discontinued EPIVIR-HBV at Week 52 and patients in the same studies who received placebo throughout the treatment course is shown in Table 7
Table 7. Posttreatment ALT Elevations in 2 Placebo-Controlled Studies in Adults With No-Active-Treatment Follow-up (Studies 1 and 3)
Abnormal Value Patients With ALT Elevation/Patients With Observationsa . EPIVIR-HBV Placebo ALTx baseline value 37/137 (27%) 22/116 (19%) ALTx baseline valueb 29/137 (21%) 9/116 (8%) ALTx baseline value and absolute ALT >500 IU/L 21/137 (15%) 8/116 (7%) ALTx baseline value 1/137 (0.7%) 1/116 (0.9%)
a Each patient may be represented in one or more category.
b Comparable to a Grade 3 toxicity in accordance with modified WHO criteria.
ULN = Upper limit of normal.
Lamivudine in Patients With HIV:
In HIV-infected patients, safety information reflects a higher dose of lamivudine (150 mg b.i.d.) than the dose used to treat chronic hepatitis B in HIV-negative patients. In clinical trials using lamivudine as part of a combination regimen for treatment of HIV infection, several clinical adverse events occurred more often in lamivudine-containing treatment arms than in comparator arms. These included nasal signs and symptoms (20% vs. 11%), dizziness (10% vs. 4%), and depressive disorders (9% vs. 4%). Pancreatitis was observed in 9 of the 2,613 adult patients (<0.5%) who received EPIVIR in controlled clinical trials. Laboratory abnormalities reported more often in lamivudine-containing arms included neutropenia and elevations of liver function tests (also more frequent in lamivudine-containing arms for a retrospective analysis of HIV/HBV dually infected patients in one study), and amylase elevations. Please see the complete prescribing information for EPIVIR Tablets and Oral Solution for more information.
Pediatric Patients With Hepatitis B:
Pediatric Patients With HIV Infection:
In early open-label studies of lamivudine in children with HIV, peripheral neuropathy and neutropenia were reported, and pancreatitis was observed in 14% to 15% of patients
Observed During Clinical Practice:
The following events have been identified during post-approval use of lamivudine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, potential causal connection to lamivudine, or a combination of these factors. Post-marketing experience with lamivudine at this time is largely limited to use in HIV-infected patients.
Digestive: Stomatitis.
Endocrine and Metabolic: Hyperglycemia.
General: Weakness.
Hemic and Lymphatic: Anemia (including pure red cell aplasia and severe anemias progressing on therapy), lymphadenopathy, splenomegaly.
Hepatic and Pancreatic: Lactic acidosis and steatosis, pancreatitis, posttreatment exacerbation of hepatitis (see WARNINGS and PRECAUTIONS).
Hypersensitivity: Anaphylaxis, urticaria.
Musculoskeletal: Rhabdomyolysis.
Nervous: Paresthesia, peripheral neuropathy.
Respiratory: Abnormal breath sounds/wheezing.
Skin: Alopecia, pruritus, rash.
OVERDOSAGE
There is no known antidote for EPIVIR. One case of an adult ingesting 6 g of EPIVIR was reported; there were not clinical signs or symptoms noted and hematologic tests remained normal. Two cases of pediatric overdose were reported in Study ACTG300. One case involved a single dose of 7mg/kg of EPIVIR; the second case involved use of 5 mg/kg of EPIVIR twoice daily for 30 days. There were no clinical signs or symptoms noted in either case. Because a negligible amount of lamivudine was removed via (4-hour) hemodialysis, continuous ambulatory peritoneal dialysis, and automated peritoneal dialysis, it is not known if continuous hemodialysis would provide clinical benefit in a lamivudine overdose event. If overdose occurs, the patient should be monitored, and standard supportive treatment applied as required.
DOSAGE & ADMINISTRATION
Adults:
The recommended oral dose of EPIVIR-HBV for treatment of chronic hepatitis B in adults is 100 mg once daily (see paragraph below and WARNINGS). Safety and effectiveness of treatment beyond 1 year have not been established and the optimum duration of treatment is not known (see PRECAUTIONS).
The formulation and dosage of lamivudine in EPIVIR-HBV are not appropriate for patients dually infected with HBV and HIV. If lamivudine is administered to such patients, the higher dosage indicated for HIV therapy should be used as part of an appropriate combination regimen, and the prescribing information for EPIVIR as well as EPIVIR-HBV should be consulted.
Pediatric Patients:
The recommended oral dose of EPIVIR-HBV for pediatric patients 2 to 17 years of age with chronic hepatitis B is 3 mg/kg once daily up to a maximum daily dose of 100 mg. Safety and effectiveness of treatment beyond 1 year have not been established and the optimum duration of treatment is not known (see PRECAUTIONS).
EPIVIR-HBV is available in a 5-mg/mL oral solution when a liquid formulation is needed. (Please see information above regarding distinctions between different lamivudine-containing products.)
Dose Adjustment:
It is recommended that doses of EPIVIR-HBV be adjusted in accordance with renal function (Table 8) (see CLINICAL PHARMACOLOGY: Special Populations).
Table 8. Adjustment of Adult Dosage of EPIVIR-HBV in Accordance With Creatinine Clearance
Creatinine ClearanceRecommended Dosage of EPIVIR-HBV(mL/min)100 mg once daily30-49100 mg first dose, then 50 mg once daily15-29100 mg first dose, then 25 mg once daily5-1435 mg first dose, then 15 mg once daily<535 mg first dose, then 10 mg once dailyNo additional dosing of EPIVIR-HBV is required after routine (4-hour) hemodialysis or peritoneal dialysis.
Although there are insufficient data to recommend a specific dose adjustment of EPIVIR-HBV in pediatric patients with renal impairment, a dose reduction should be considered.
HOW SUPPLIED
EPIVIR-HBV Tablets, 100 mg, are butterscotch-colored, film-coated, biconvex, capsule-shaped tablets imprinted withGX CG5on one side.
Bottles of 60 tablets (NDC 0173-0662-00) with child-resistant closures.
Store at 25(77excursions permitted to 15to 30(59to 86[see USP Controlled Room Temperature].
REFERENCES
EPIVIR-HBV is a registered trademark of GlaxoSmithKline.
The other brands listed are trademarks of their respective owners and are not trademarks of GlaxoSmithKline. The makers of these brands are not affiliated with and do not endorse GlaxoSmithKline or its products.
GlaxoSmithKline
Research Triangle Park, NC 27709
Manufactured under agreement from
Shire Pharmaceuticals Group plc
Basingstoke, UK
GlaxoSmithKline. All rights reserved.
INACTIVE INGREDIENT
HYPROMELLOSES
MAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
POLYETHYLENE GLYCOL
POLYSORBATE 80
SODIUM STARCH GLYCOLATE TYPE A POTATO
TITANIUM DIOXIDE
| EPIVIR
lamivudine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |