Label: DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH- benzocaine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 67659-410-03, 67659-410-04 - Packager: Team Technologies, Inc
- This is a repackaged label.
- Source NDC Code(s): 60630-077
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Allergy alert: do not use this product if you have a history of allery to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product . avoid contact with the eyes . do not exceed recommended dosage . do not use for more than 7 days unless directed by a doctor/dentist
-
DOSAGE & ADMINISTRATION
Directions. dip applicator into liquid
Adults and children 2 years of age and older: Apply a small amount of product to the affected area. Use up to 4 times daily or as directed by a dentist or doctor.
Children under 12 years of age: Should be supervised in the use of this product.
Children under 2 years of age: Ask a dentist or doctor.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
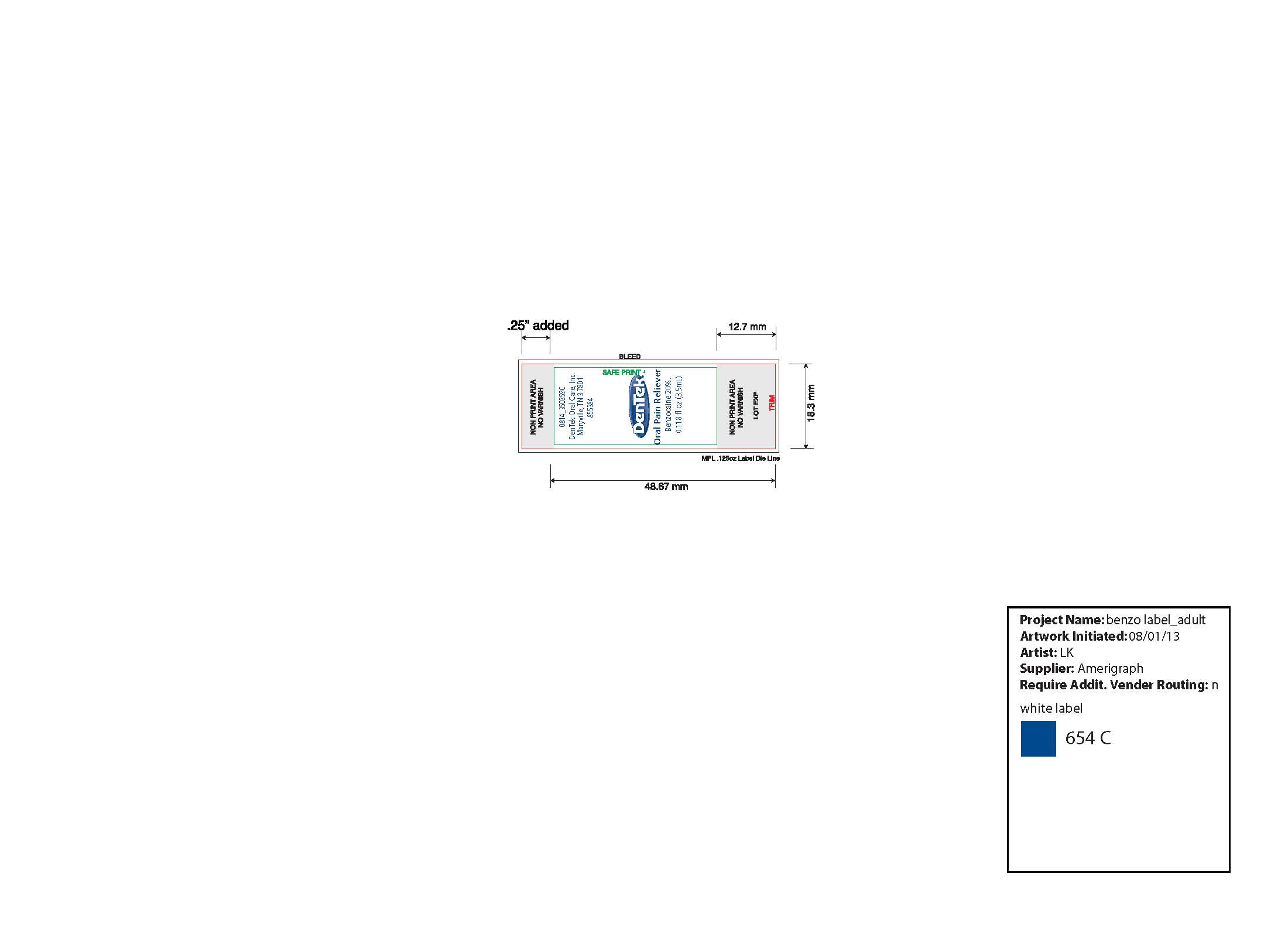
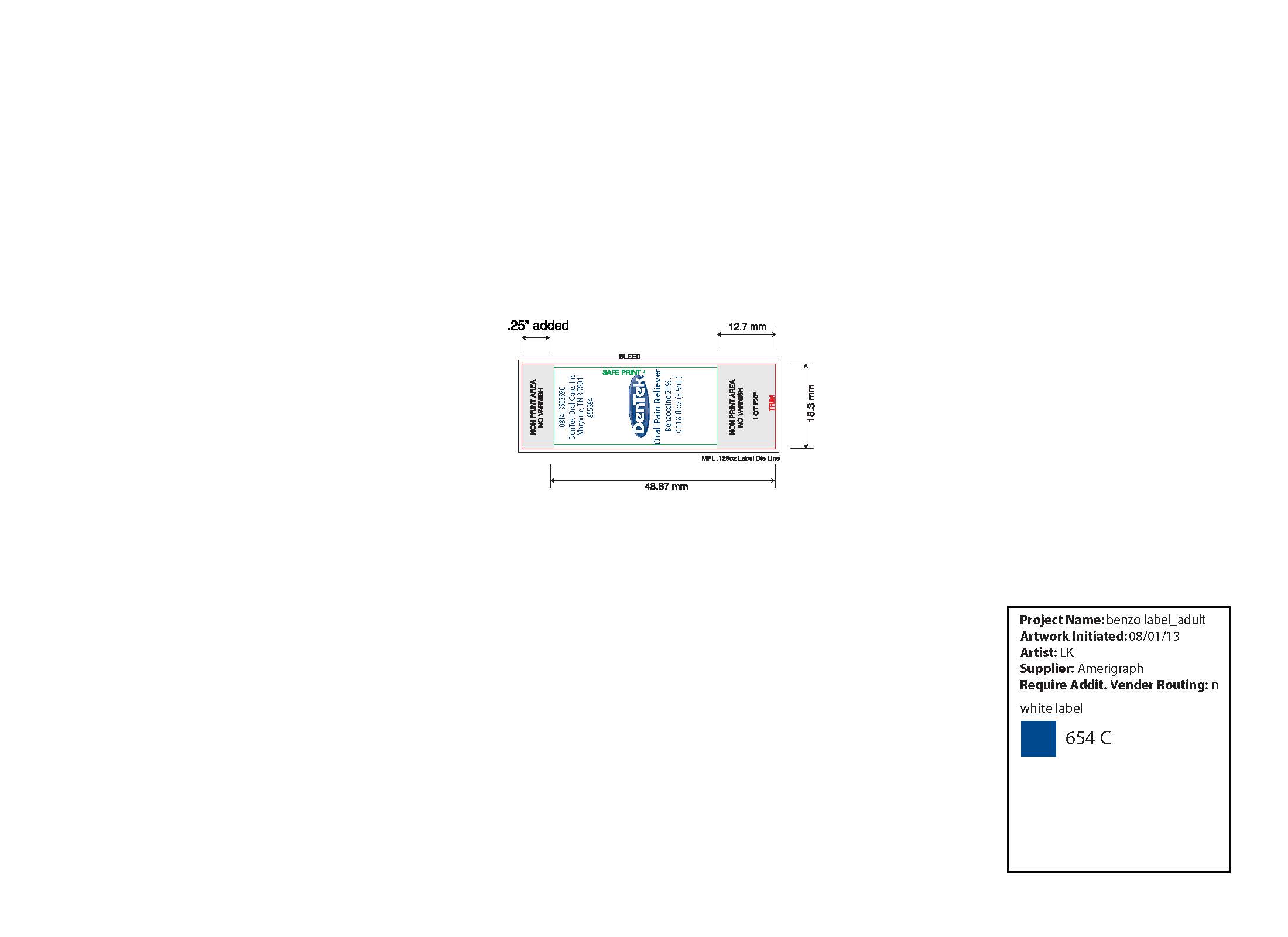
- DenTek Instant Pain Relief Inner Vial Label
- DenTek Instant Pain Relief Outer Carton Label
-
INGREDIENTS AND APPEARANCE
DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67659-410(NDC:60630-077) Route of Administration DENTAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) 10 mg in 1 mL PEPPERMINT OIL (UNII: AV092KU4JH) 2.2 mg in 1 mL SPEARMINT OIL (UNII: C3M81465G5) 3.3 mg in 1 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 784.5 mg in 1 mL Product Characteristics Color yellow (colorless to light yellow liquid) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67659-410-04 1 in 1 CARTON 07/31/2015 1 NDC:67659-410-03 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/20/2002 Labeler - Team Technologies, Inc (192339703) Registrant - Team Technologies, Inc (192339703) Establishment Name Address ID/FEI Business Operations Team Technologies, Inc 079527756 relabel(67659-410) , repack(67659-410)