APLICARE POVIDONE IODINE- povidone iodine gel
Aplicare Products, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
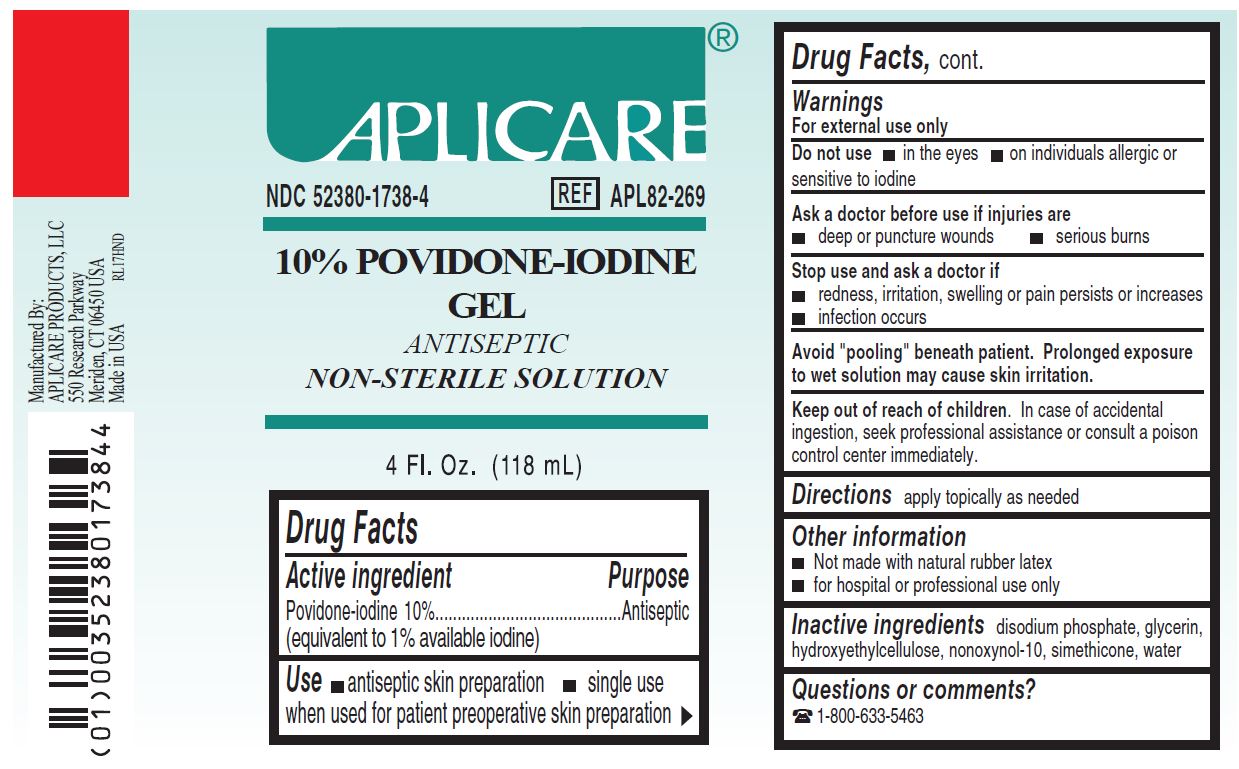
1738 Aplicare Povidone Iodine Gel, 10%
Warnings
- For external use only
- Avoid "pooling" beneath patient. Prolonged exposure to wet solution may cause skin irritation.
Inactive ingredients
disodium phosphate, glycerin, hydroxyethylcellulose, nonoxynol-10, simethicone, water
| APLICARE POVIDONE IODINE
povidone iodine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aplicare Products, LLC (081054904) |
| Registrant - Medline Industries, LP (025460908) |
Revised: 12/2021
Document Id: d2195149-8a70-1445-e053-2995a90aec96
Set id: 3a7874fb-e664-4e2c-b453-1aeee4832add
Version: 8
Effective Time: 20211201
Aplicare Products, LLC