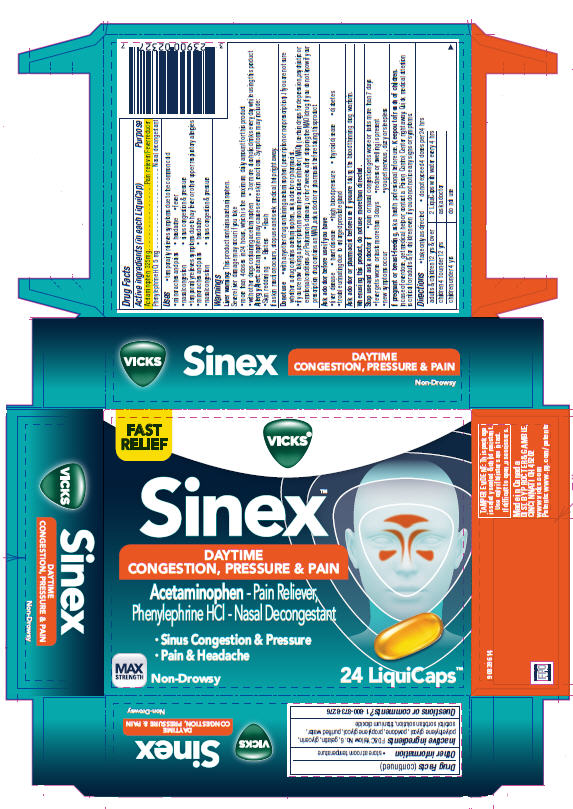
VICKS SINEX- acetaminophen and phenylephrine hydrochloride capsule, liquid filled
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vicks
® Sinex™
Daytime Congestion, Pressure & Pain
Uses
- temporarily relieves symptoms due to the common cold
- minor aches and pains
- headache
- fever
- nasal congestion
- sinus congestion & pressure
- temporarily relieves symptoms due to hay fever or other upper respiratory allergies
- minor aches and pains
- headache
- nasal congestion
- sinus congestion & pressure
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 4 doses in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. - if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to enlarged prostate gland
Directions
- take only as directed
- do not exceed 4 doses per 24 hrs
| adults & children 12 yrs & over | 2 LiquiCaps with water every 4 hrs |
| children 4 to under 12 yrs | ask a doctor |
| children under 4 yrs | do not use |
| VICKS SINEX
acetaminophen and phenylephrine hydrochloride capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 7/2018
Document Id: 72502742-43ec-e895-e053-2991aa0a6075
Set id: 3a362bd9-da7e-58cd-e054-00144ff8d46c
Version: 6
Effective Time: 20180731
The Procter & Gamble Manufacturing Company