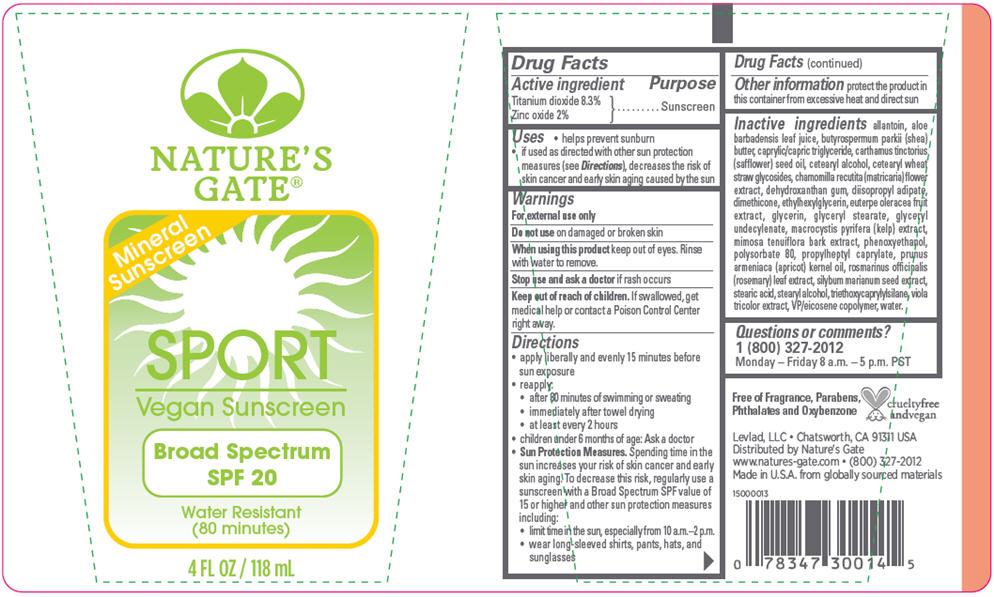
NATURES GATE SPORT SPF 20 MINERAL- titanium dioxide and zinc oxide lotion
Levlad, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Titanium dioxide 8.3%
Zinc oxide 2%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally and evenly 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Other information
- protect the product in this container from excessive heat and direct sun
Inactive ingredients
allantoin, aloe barbadensis leaf juice, butyrospermum parkii (shea) butter, caprylic/capric triglyceride, carthamus tinctorius (safflower) seed oil, cetearyl alcohol, cetearyl wheat straw glycosides, chamomilla recutita (matricaria) flower extract, dehydroxanthan gum, diisopropyl adipate, dimethicone, ethylhexylglycerin, euterpe oleracea (açai) fruit extract, glycerin, glyceryl stearate, glyceryl undecylenate, macrocystis pyrifera extract, mimosa tenuiflora bark extract, phenoxyethanol, polysorbate 80, propylheptyl caprylate, prunus armeniaca (apricot) kernel oil, rosmarinus officinalis (rosemary) leaf extract, silybum marianum (milk thistle) seed extract, stearic acid, stearyl alcohol, triethoxycaprylylsilane, viola tricolor (wild pansy) extract, VP/eicosene copolymer, water.
Questions or comments?
1 (800) 327-2012 Monday – Friday 8 a.m. – 5 p.m. PST
Distributed by Nature's Gate
Chatsworth, CA 91311
PRINCIPAL DISPLAY PANEL - 118 mL Tube Label
NATURE'S
GATE®
Mineral
Sunscreen
SPORT
Vegan Sunscreen
Broad Spectrum
SPF 20
Water Resistant
(80 minutes)
4 FL OZ / 118 mL