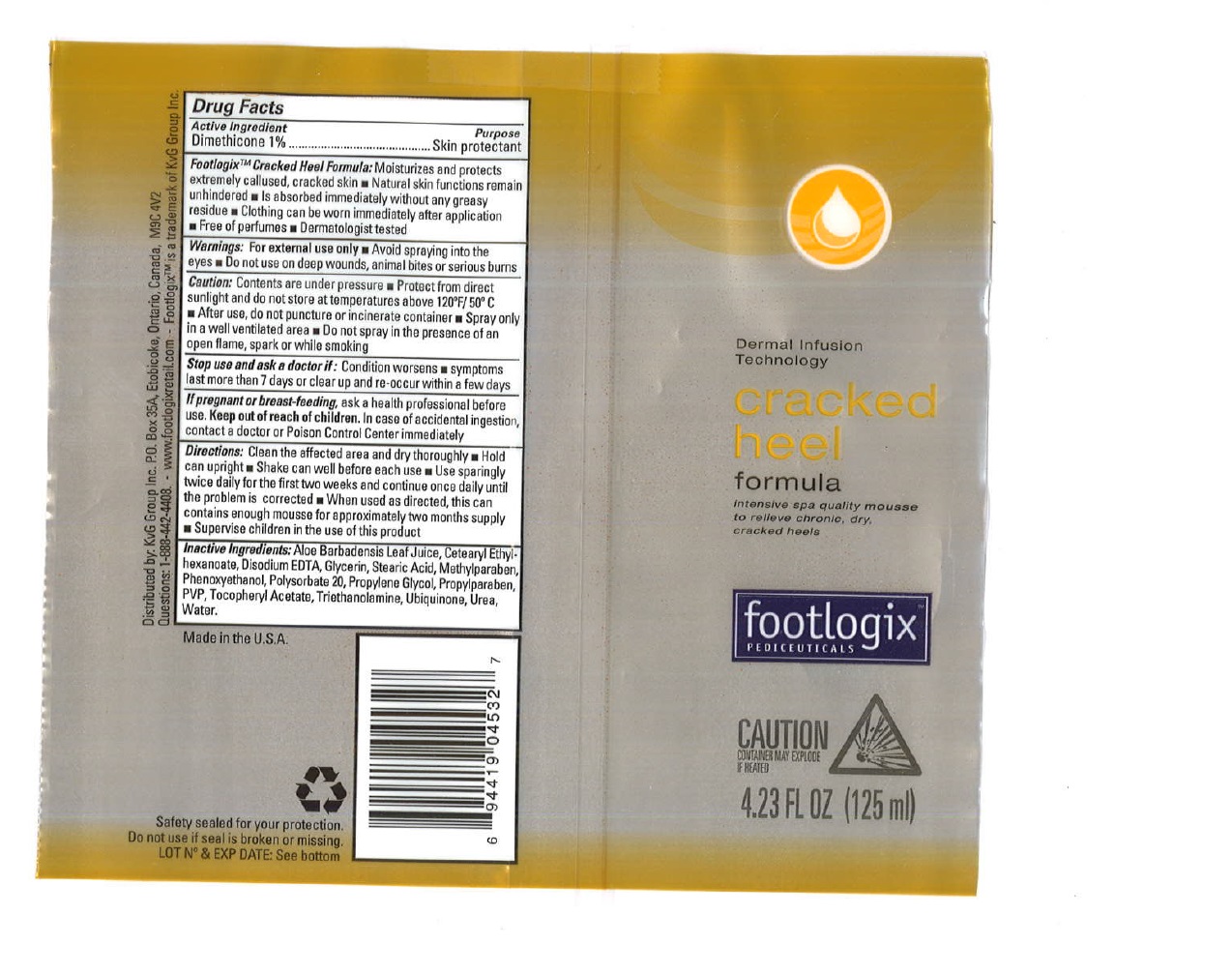
Label: KVG GROUP, INC. FXP CRACKED HEEL FORMULA- dimethicone spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 13630-0114-3 - Packager: Prime Packaging Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Footlogix TM
-
Warnings
For external use only
- Avoid spraying into the eyes
Caution:
Contents are under pressure
- Protect from direct sunlight and do not store at temperatures above 120ºF / 50ºC
- After use, do not puncture or incinerate container
- Spray only in a well ventilated area
- Do not spray in the presence of an open flame, spark or while smoking
-
Directions:
Clean the affected area and dry thoroughly
- Hold can upright
- Shake can well before each use
- Use sparingly twice daily for the first two weeks and continue once daily until the problem is corrected
- When used as directed, this can contains enough mousse for approximately two months supply
- Supervise children in the use of this product
- Inactive Ingredients
- FXP Cracked Heel Formula
-
INGREDIENTS AND APPEARANCE
KVG GROUP, INC. FXP CRACKED HEEL FORMULA
dimethicone sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13630-0114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 10.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTANE (UNII: 6LV4FOR43R) PROPANE (UNII: T75W9911L6) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TROLAMINE (UNII: 9O3K93S3TK) UREA (UNII: 8W8T17847W) POLYSORBATE 20 (UNII: 7T1F30V5YH) STEARIC ACID (UNII: 4ELV7Z65AP) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) UBIDECARENONE (UNII: EJ27X76M46) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13630-0114-3 125 mL in 1 CAN; Type 0: Not a Combination Product 05/21/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 05/21/2014 Labeler - Prime Packaging Inc. (805987059) Registrant - Prime Packaging Inc. (805987059) Establishment Name Address ID/FEI Business Operations Prime Enterprises Inc 101946028 analysis(13630-0114) , manufacture(13630-0114) Establishment Name Address ID/FEI Business Operations Prime Packaging Inc. 805987059 pack(13630-0114) , label(13630-0114)