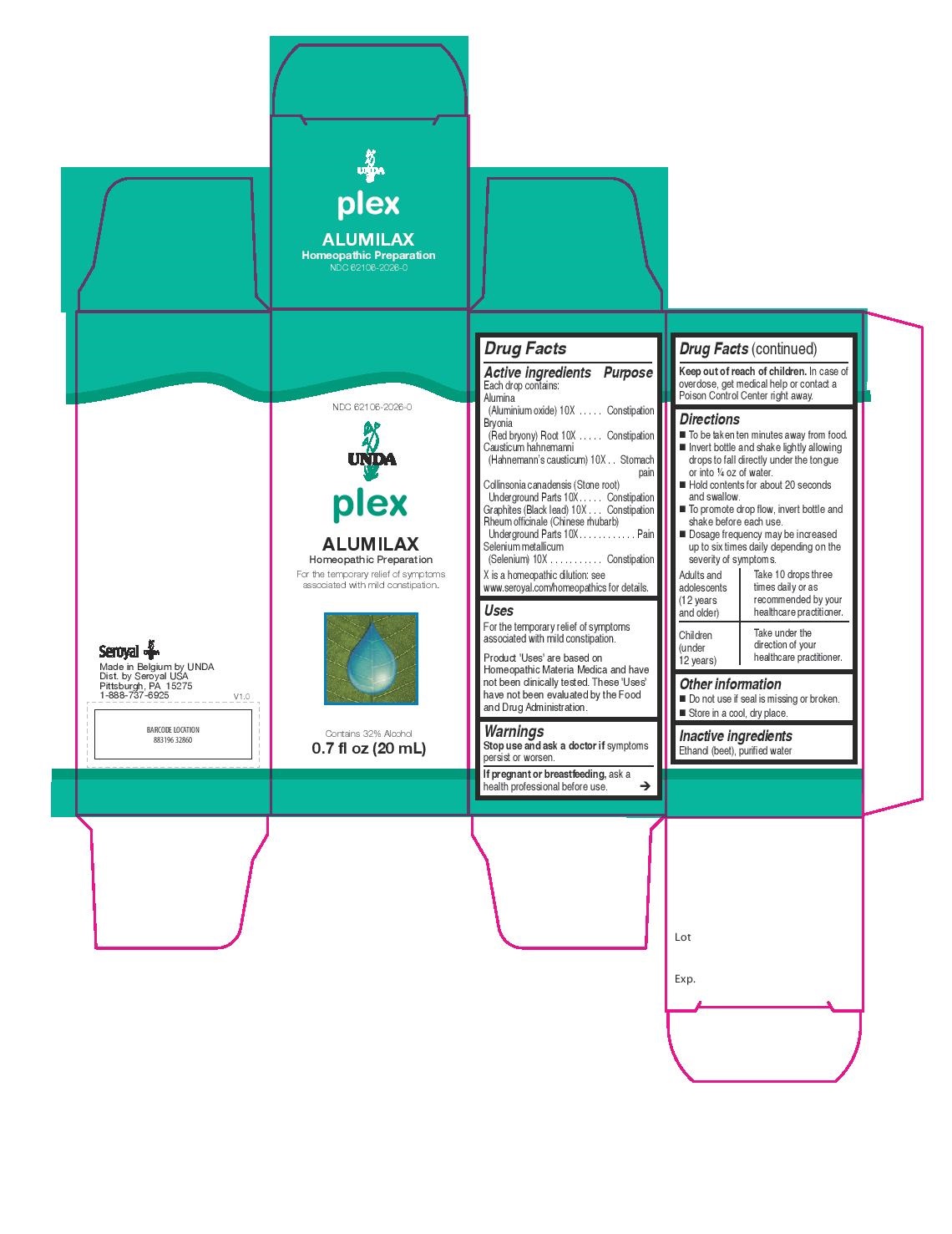
ALUMILAX- alumina, bryonia, causticum hahnemanni, collinsonia canadensis, graphites, rheum officinale, selenium metallicum liquid
Seroyal USA
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ALUMILAX
Active ingredients
Each drop contains:
Alumina (Aluminium oxide) 10X
Bryonia (Red bryony) Root 10X
Causticum hahnemanni (Hahnemann’s causticum) 10X
Collinsonia canadensis (Stone root) Underground Parts 10X
Graphites (Black lead) 10X
Rheum officinale (Chinese rhubarb) Underground Parts 10X
Selenium metallicum (Selenium) 10X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
To be taken ten minutes away from food.
Invert bottle and shake lightly allowing drops to fall directly under the tongue or into ¼ oz of water.
Hold contents for about 20 seconds and swallow.
To promote drop flow, invert bottle and shake before each use.
Dosage frequency may be increased up to six times daily depending on the severity of symptoms.
Adults and adolescents (12 years and older)
Take 10 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with mild constipation.
Directions
To be taken ten minutes away from food.
Invert bottle and shake lightly allowing drops to fall directly under the tongue or into ¼ oz of water.
Hold contents for about 20 seconds and swallow.
To promote drop flow, invert bottle and shake before each use.
Dosage frequency may be increased up to six times daily depending on the severity of symptoms.
Adults and adolescents (12 years and older)
Take 10 drops three times daily or as recommended by your
Children (under 12 years)
Take under the direction of your healthcare practitioner.
| ALUMILAX
alumina, bryonia, causticum hahnemanni, collinsonia canadensis, graphites, rheum officinale, selenium metallicum liquid |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Seroyal USA (018361118) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SAN’UP | 401010287 | manufacture(62106-2026) | |