VISINE TOTALITY MULTI SYMPTOM RELIEF- glycerin, hypromelloses, polyethylene glycol 400, tetrahydrozoline hydrochloride, and zinc sulfate liquid
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Visine® Totality® Multi-Symptom Relief
Uses
- for the relief of discomfort and redness of the eye due to minor eye irritations
- relieves dryness of the eye
- for the temporary relief of burning and irritation due to exposure to wind or sun
- for protection against further irritation
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Directions
- put 1 to 2 drops in the affected eye(s) up to 4 times a daily
- children under 6 years of age: ask a doctor
Other information
- some users may experience a brief tingling sensation
- store at 20° to 25°C (68° to 77°F)
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium chloride, sodium citrate
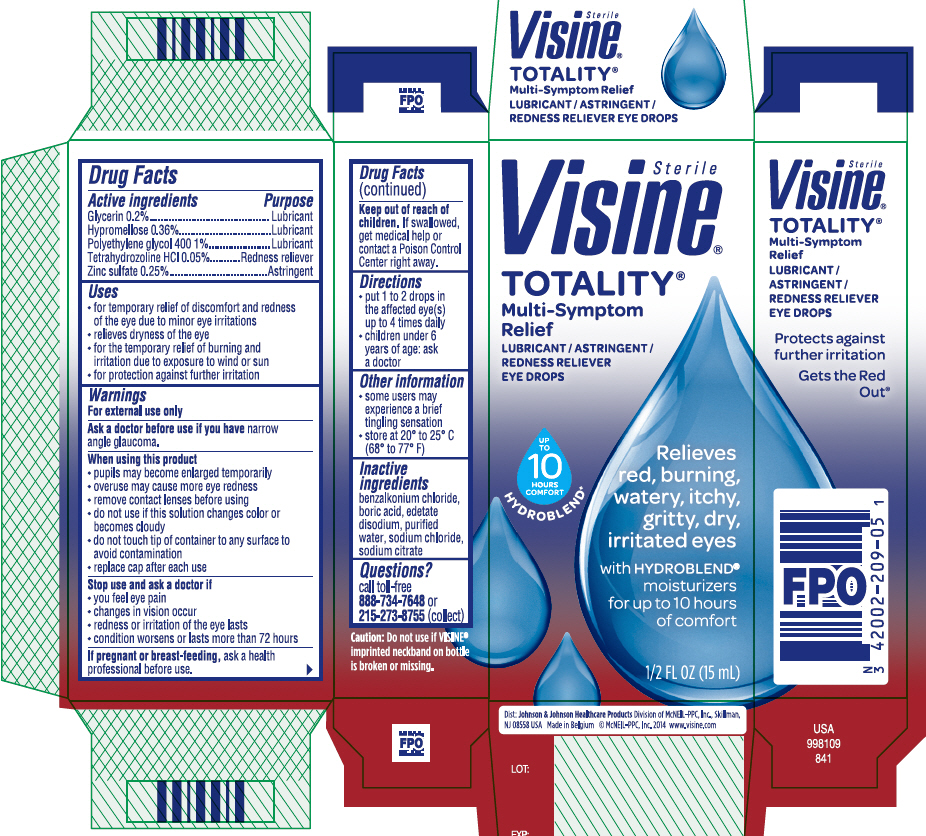
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
Sterile
Visine®
TOTALITY®
Multi-Symptom
Relief
LUBRICANT / ASTRINGENT /
REDNESS RELIEVER
EYE DROPS
UP
TO
10
HOURS
COMFORT
HYDROBLEND®
Relieves
red, burning,
watery, itchy,
gritty, dry,
irritated eyes
with HYDROBLEND®
moisturizers
for up to 10 hours
of comfort
1/2 FL OZ (15mL)
| VISINE TOTALITY
MULTI SYMPTOM RELIEF
glycerin, hypromelloses, polyethylene glycol 400, tetrahydrozoline hydrochloride, and zinc sulfate liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |