Label: BLEOMYCIN injection, powder, lyophilized, for solution
- NDC Code(s): 70121-1567-1
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
IMPORTANT
DRUG INFORMATION
July 5, 2016
Re: Temporary importation of Bleomycin Sulfate Powder for Injection, 15,000 International Units (IU) to address drug shortage issue
Dear Healthcare Professional,
IMPORTANT NOTE:
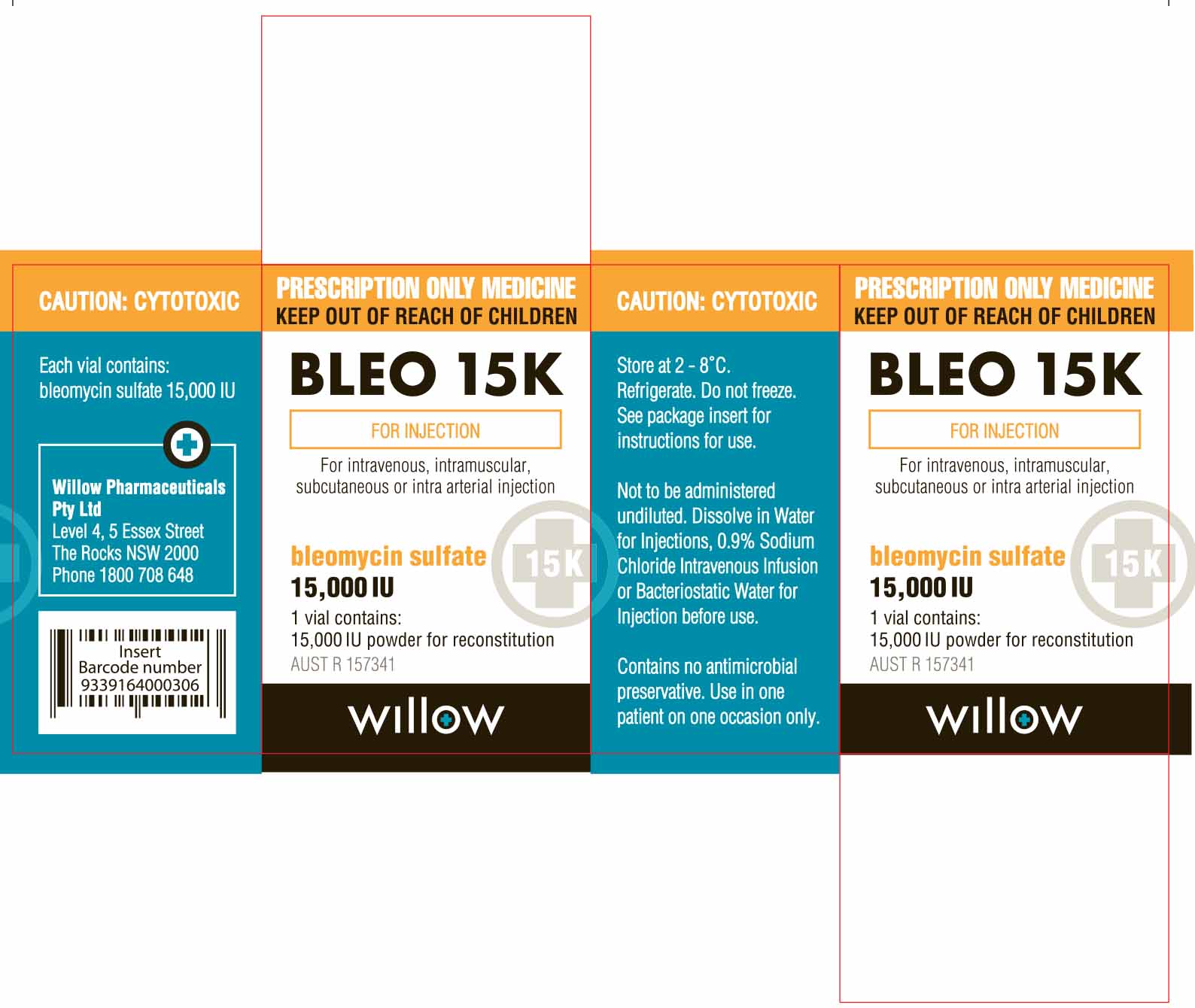
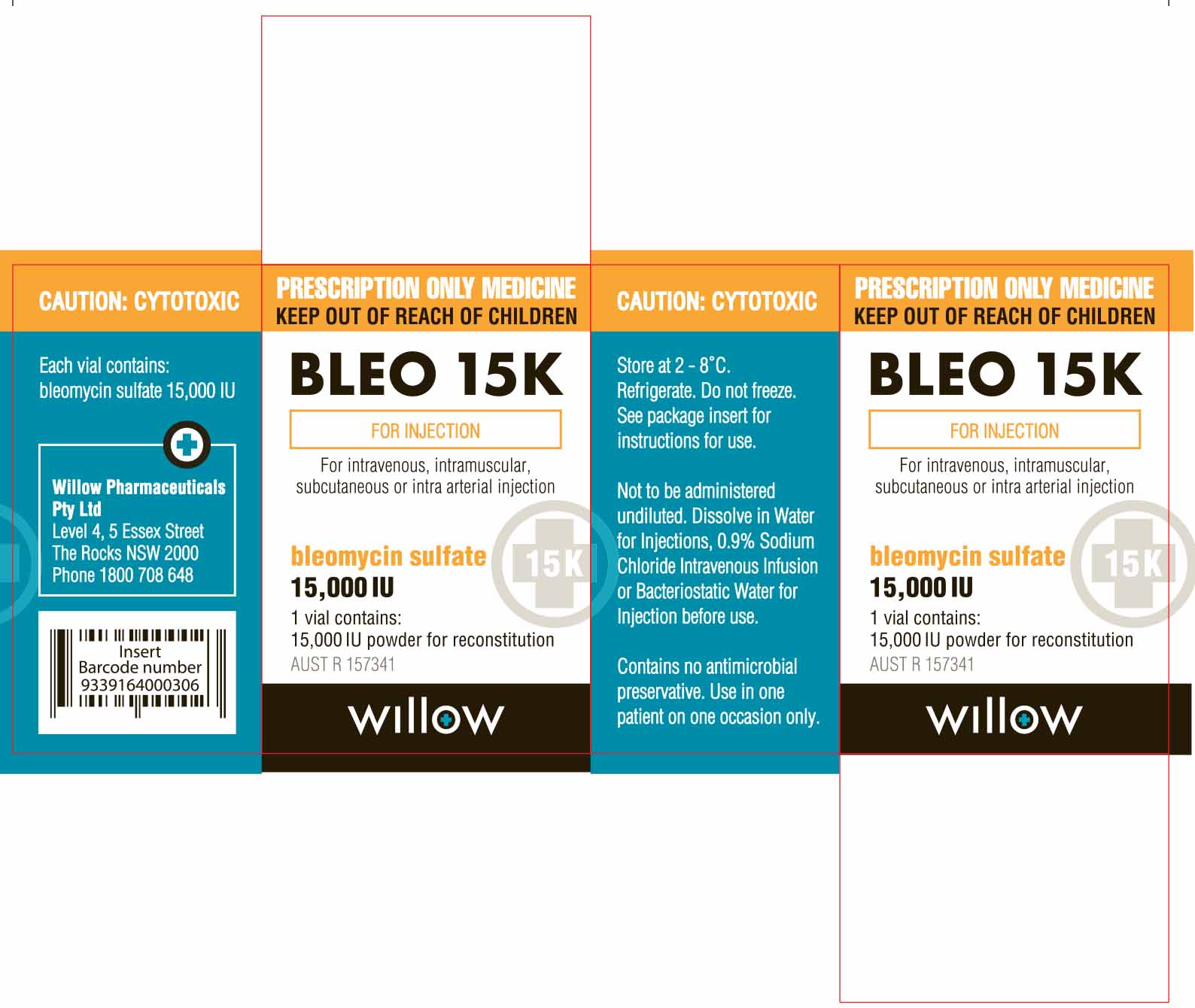
Each vial of Amneal Biosciences’ product contains 15,000 IU of Bleomycin Sulfate Powder for Injection, which is equivalent to Bleomycin Sulfate USP 15 units (1000 IU of Bleomycin Sulfate Powder for Injection = 1 unit of Bleomycin Sulfate USP). Prescribers and pharmacists must be alert to this difference in labeled units in order to prevent medication errors. Appropriate quality assurance measures should be enacted to reduce the risk of medication errors.
Due to the current critical shortage of Bleomycin Sulfate for Injection, USP, 15 units and 30 units per vial in the United States (U.S.) market, Amneal Biosciences is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of Bleomycin Sulfate for Injection, USP, 15 units per vial.
Amneal Biosciences has initiated temporary importation of a non-FDA approved product, Bleomycin Sulfate Powder for Injection 15,000 IU per vial to the U.S. market: lot numbers GE50604, GE60003, GE60012. The Bleomycin Sulfate Powder for Injection 15,000 IU product being distributed by Amneal Biosciences was manufactured by Cipla Ltd. from its FDA-inspected facility in Verna, Goa, India, for Amneal Pharma Australia Pty Ltd and was sold in Australia under the WIllow Pharmaceutical label.
At this time, no other entity except Amneal Biosciences is authorized by the FDA to import or distribute Amneal Pharma Australia Pty Ltd.’s Bleomycin Sulfate Powder for Injection 15,000 IU product. FDA has not approved the Bleomycin Sulfate Powder for Injection 15,000 IU product being distributed by Amneal Biosciences in the United States.
Amneal Biosciences’ Bleomycin Sulfate Powder for Injection 15,000 IU product contains the same active ingredient, bleomycin sulfate, in the same strength as the U.S. FDA-approved Bleomycin Sulfate for Injection, USP, 15 units per vial.
In the U.S., the labeling for Bleomycin Sulfate for Injection includes the following indications: i) squamous cell carcinoma; ii) non-Hodgkin lymphoma; iii) testicular carcinoma; iv) Hodgkin lymphoma; v) malignant pleural effusion.
Bleomycin Sulfate Powder for Injection 15,000 IU product being distributed by Amneal Biosciences is approved in Australia and is dispensed with a Medication Guide. The information on the medication guide differs from the full prescribing information for the current U.S. FDA reference listed drug (RLD). Two key differences between the two package inserts is that the U.S. FDA-approved label uses different units from the Amneal Biosciences label and the Amneal Australia label does not include an indication for malignant pleural effusions. Additionally, the U.S. FDA-approved label provides weight-based dosing information as well as body surface area-based dosage, whereas the imported product label contains only BSA-based dosing information.
This letter is not intended as a complete description of the benefits and risks related to the use of Bleomycin Sulfate Powder for Injection 15,000 IU. Please refer to the package insert for the U.S. FDA-approved Bleomycin Sulfate for Injection, USP for full prescribing information.
Please note that the barcode used for Amneal Biosciences’ Bleomycin Sulfate Powder for Injection, 15,000 IU product may not be appropriately recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
The Bleomycin Sulfate Powder for Injection 15,000 IU product distributed by Amneal Biosciences should be handled exactly as you have handled the U.S. FDA-approved Bleomycin Sulfate for Injection, USP. Refrigerate unopened vials at 2°C to 8°C (36°F to 46°F). The Bleomycin Sulfate Powder for Injection 15,000 IU product distributed by Amneal Biosciences should not be reconstituted or diluted with D5W or other dextrose-containing diluents.
To order Bleomycin Sulfate Powder for Injection, 15,000 IU product distributed by Amneal Biosciences, health care professionals can order the product from their wholesaler/distributor of choice.
To report adverse events or medication errors, or for answers to medical and technical inquiries for patients who have used Amneal Biosciences’ Bleomycin Sulfate Powder for Injection, 15,000 IU product, please contact Amneal Biosciences Drug Safety by telephone at 1-855-266-3251 Monday through Friday from 9:00-5:30 EST, or email at biosciencesdrugsafety@amneal.com.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, or regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
For full U.S. FDA reference listed drug (RLD) prescribing information for Bleomycin Sulfate for Injection, USP, including the BOXED WARNING, please visit:
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5806c40-12ce-48e3-8abd-9f8997ef4428
For full prescribing information for Amneal Bioscience’s Bleomycin Sulfate Powder for Injection 15,000 IU product, please visit:
Sincerely,
Charles D. Lucarelli
President
Amneal Biosciences LLC
-
DESCRIPTION
Bleomycin sulfate is a white or yellowish white or cream colored amorphous hygroscopic powder. It is very soluble in water slightly soluble in dehydrated alcohol, and practically insoluble in acetone and ether.
It is a purified mixture of glycopeptides produced by a fermentation process employing the actinomycetes Streptoverticillium species. The bleomycin mixture contains predominantly the A2, and B2, peptides. When reconstituted in water for injection, the pH of the solution is approximately 5. Each vial contains 55 to 70% of bleomycin A2, and 25 to 32% of bleomycin B2.
Bleomycin injection contains the excipients: hydrochloric acid and sodium hydroxide, both for pH adjustment.
-
CLINICAL PHARMACOLOGY
Although the precise mechanism of action of bleomycin is not fully known, it is thought that the primary action is to produce single and double strand breaks in DNA, leading to inhibition of cell division and growth, and inhibition of DNA synthesis in the cells. Bleomycin is probably most effective against cells in the M and G2 (premitotic) phase of the cell cycle. Bleomycin has not been shown to have an immunosuppressive effect in vitro and shows no significant inhibition of immune response in patients treated with the drug. Bleomycin inactivating enzyme has been detected in both normal and malignant cells and is particularly prominent in
Pharmacokinetics
Absorption
Bleomycin is well absorbed in animals upon parenteral administration. Intramuscular injection of 15 units in humans resulted in a maximum serum concentration of 1 mU/mL 30 minutes after administration. Intravenous injection of 15 units in humans resulted in a maximum serum
concentration of 3.3 mU/mL.
Distribution
In mice, bleomycin diffusing from the blood produces high concentrations in the skin, lungs, kidneys, peritoneum, lymphatic system and susceptible tumour tissue if present. Bleomycin crosses the placenta, but does not cross the blood brain barrier. Equilibrium dialysis and gel permeation experiments suggest that less than 1.0% of the drug is protein bound after incubation with normal human serum in vitro.
Metabolism
The majority of a bleomycin dose is not readily metabolised. The highest rate of metabolism occurs in the liver and gastrointestinal tract. A lower rate of metabolism also occurs in skin, lungs, kidneys, muscle and serum. The products of bleomycin metabolism are not known.
Excretion
Bleomycin is primarily excreted in the urine. After intravenous injection an average of 40% of the administered dose is recovered unchanged in the urine within 24 hours. After intramuscular injection 20% is recovered in the urine after six hours. The plasma half-lives have varied from 15 to 60 minutes in patients with normal renal function following intravenous administration. The serum half-life is prolonged in patients with renal dysfunction. In one patient with severe renal dysfunction the biological half-life was 21 hours when the creatinine clearance was 10.7 mL/minute, and 13 hours when the creatinine clearance was 15.2 mL/minute. There were undetectable serum levels of bleomycin 72 hours after the intravenous dose.
Interactions with other medicines
-Pharmacodynamic interactions
Anaesthetics, general and oxygen. Use in patients previously treated with bleomycin may result in rapid pulmonary deterioration, since bleomycin causes sensitisation of lung tissue to oxygen.
Radiation therapy. Radiation therapy, especially to the chest area, either prior to, during or after bleomycin therapy may result in increased bleomycin toxicity. Dosage adjustment may be necessary.
Antineoplastic agents. Concurrent use may result in increased bleomycin toxicity, or in occurrence of pulmonary toxicity at lower doses of bleomycin (see Precautions).
Combination therapy. Pulmonary toxicity may be observed at lower doses of bleomycin when bleomycin is administered as part of a multidrug treatment regimen. Patients should be closely monitored for signs of pulmonary toxicity (see Precautions).
Granulocyte colony stimulating factor. It has been suggested that concomitant administration of G-CSF and bleomycin may increase the risk of bleomycin induced pulmonary toxicity, especially at higher doses, although this has not been confirmed in clinical trials. If G-CSF is added to bleomycin containing treatment regimens, patients should be closely observed for signs of pulmonary toxicity (see Precautions).
-Pharmacokinetic interactions
Cisplatin. Cisplatin induced renal function impairment may result in delayed clearance and bleomycin toxicity even at low doses. An increased incidence of bleomycin induced pulmonary toxicity has been observed when these two agents are administered as part of an antineoplastic treatment regimen. Dosage reduction may be required (see Precautions).
Digoxin. Serum levels of digoxin may be reduced and its actions may be decreased. It is thought that drug induced alterations of the intestinal mucosa may be involved in the reduced gastrointestinal absorption.
Phenytoin. Serum concentrations of phenytoin may be decreased due to decreased absorption or increased metabolism of phenytoin.
-
INDICATIONS AND USAGE
Palliation and treatment adjutant to surgery and radiation therapy of the following neoplasms:
- Squamous cell carcinoma of the skin, head and neck, and esophagus (primary indication).
- Squamous cell carcinoma of the larynx, penis and uterine cervix.
- Squamous cell carcinoma of the bronchus (response infrequent).
- Ch0riocarcinoma and embryonal cell carcinoma of the testis.
- Advanced Hodgkin’s disease and Other lymphomas.
- Mycosis fungoides.
Note. Use of bleomycin after radiation therapy is less successful than use before radiation therapy. Bleomycin is bone marrow sparing and may be used when other cytotoxic agents are contraindicated.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
It is recommended that bleomycin be administered under the supervision of a qualified doctor experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available. Patients receiving bleomycin must be Observed carefully and frequently during and after therapy.
After injection, bleomycin is readily absorbed and distributed in the body, particularly in the skin, lungs and any susceptible tumor tissue, leading to possible skin and pulmonary toxicity, as well as antitumor activity.
Pulmonary Toxicity
No single predictive monitoring test for bleomycin induced pulmonary toxicity has been identified. Frequent physical examinations should be undertaken. Cough, basal rales and pleuritic chest pain are frequent first signs of toxicity. Dyspnea is usually the first symptom. It pulmonary changes are noted, treatment should be discontinued until it can be determined whether the cause is drug related. Pulmonary function test (especially total lung volume (TL\/) and forced vital capacity (FVC)) may be of value in detecting early lung changes, although these are not always predictive of subsequent toxicity. It has been suggested that bleomycin should be discontinued it F\/C decreases rapidly. Baseline and subsequent monthly evaluation of carbon monoxide diffusion capacity (D\ ) are also recommended, and bleomycin should be discontinued when the DLI is less than 30 to 35% of the pretreatment value.
The most commonly recommended method of monitoring the Onset of pulmonary toxicity is weekly chest X-rays, which should be continued up to four weeks after completion of treatment. However, nigh resolution computer tomography is a more sensitive method of detection.
Other proposed methods of monitoring pulmonary toxicity include 99mTechnetium scans and measurement of erythrocyte sedimentation rate (ESR), which has been found to increase prior to the development of symptomatic toxicity. However, the usefulness of these methods as predictors of development of toxicity have not been proven in clinical practice
Anesthesia
Because of bleomycin's effects on lung tissue, patient who have received the drug are at increased risk of developing pulmonary toxicity when oxygen is administered during surgery. Long exposure to very high concentrations of oxygen is a known cause of lung damage, but after administration of bleomycin, lung damage can occur at oxygen concentrations lower than those usually considered safe.
Therefore, to minimize the risk in patients undergoing surgery who have received bleomycin, the following is recommended:
- FI 0, (fraction of inspired oxygen) concentration should be maintained at approximately that of room air (25%) during surgery and the postoperative period;
- Fluid replacement should be carefully monitored, with emphasis on administration of colloid rather than crystalloid.
Pneumonitis
Pneumonitis due to bleomycin has been treated with corticosteroids in an effort to prevent progression to pulmonary fibrosis. Infectious pneumonitis should receive appropriate antibiotic therapy.
Lung Cancer
Bleomycin should be used with extreme caution in patient with lung cancer as these patients show an increased incidence of pulmonary toxicity.
Compromised pulmonary function due to disease other than malignancy
Bleomycin should be used with extreme caution in patient with compromised pulmonary function as pulmonary toxicity may be particularly dangerous in these patients
(see CONTRAINDICATIONS).
Previous cytotoxic or radiation therapy (especially chest radiation); smokers
Bleomycin should be used with caution in patients who have had previous cytotoxic drug therapy or radiation therapy (especially chest irradiation), and in patient who smoke, since the risk of pulmonary toxicity may be increased in these patients.
Cisplatin
Cisplatin induced renal function impairment may result in delayed clearance and bleomycin toxicity even at low doses. An increased incidence of bleomycin induced pulmonary toxicity has been observed when these two agents are administered as part of an antineoplastic treatment regimen.
Dosage reduction may be required (see INTERACTIONS WITH OTHER MEDICINES).
Granulocyte colony stimulating factor
It has been suggested that concomitant administration of granulocyte colony stimulating factor (G-CSF) and bleomycin may increase the risk of bleomycin induced pulmonary toxicity, especially at higher doses, although this has not been confirmed in clinical trials. If G-CSF is added to bleomycin containing
treatment regimens, patients should be closely observed for signs of pulmonary toxicity (see INTERACTIONS WITH OTHER MEDICINES).
Combination Therapy
Pulmonary toxicity may be observed at lower doses of bleomycin when bleomycin is administered as part of a multidrug treatment regimen. Patients should be closely monitored for signs of pulmonary toxicity (see INTERACTIONS WITH OTHER MEDICINES).
Cumulative Dose
Pulmonary toxicity is more common in patients receiving a total dose of more than 400,000 UI.
Renal or Hepatic Toxicity
Renal or hepatic toxicity, beginning as deterioration in renal or liver function tests, have been reported infrequently. These toxicities may occur, however, at any time after initiation of therapy.
Use in renally impaired patients
Bleomycin should be used with extreme caution in patients with severely impaired renal function (see DOSAGE AND ADMINISTRATION, Impaired renal function).
Idiosyncratic Reactions
Idiosyncratic reactions similar to anaphylaxis have been reported in 1% of patients treated with bleomycin (5% of lymphoma patients). Since these usually occur after the first or second dose, careful monitoring is essential after these doses.
Lymphoma Patients
All lymphoma patients should receive test doses of bleomycin before initiating full dose therapy. (See ADVERSE EFFECTS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Bleomycin is mutagenic in both in vitro and in vivo test systems. It is not known whether bleomycin is a carcinogenic in humans. However, an increased incidence of Modular hyperplasia was noted in F344/N male rats with lung cancer induced by nitrosamines, after bleomycin treatment. In another study where bleomycin was administered subcutaneously to rats at a dose of 0.35 mg/kg weekly (or about 30% the recommended human dose), necropsy findings included dose related injection site fibrosarcomas and various renal tumors. The effects of bleomycin on fertility are not known.
Use in pregnancy Category D:
Bleomycin has caused, is suspected to have caused or may be expected to cause an increased incidence of human fetal malformations or irreversible damage. It may also have adverse pharmacological effects
-
ADVERSE REACTIONS
Severe or life-threatening reactions
Pulmonary toxicity. The most serious toxicity of bleomycin is a subacute or chronic pneumonitis that progresses to interstitial fibrosis and may be fatal. This occurs in approximately 10% of treated patients, about 1% of whom have died of pulmonary fibrosis. Pulmonary toxicity is both age and dose related, being more common in patient over 70 years of age and in those receiving over 400,000 IU total dose. This toxicity, however, is unpredictable and has been seen occasionally in young patients receiving low doses. Also, when used in combination with Other antineoplastic agents, pulmonary toxicities may occur at lower doses.
This toxicity is frequently seen in those with underlying lung disease such as emphysema and in those previously treated with pulmonary or mediastinal irradiation.
The identification of patient with pulmonary toxicity due to bleomycin has been extremely difficult. The clinical symptoms and X-ray findings of bleomycin pulmonary toxicity are not easily distinguished from other syndromes commonly observed in cancer patient, including progressive metastatic tumor (especially lymphangitic tumor), infectious processes such as Pneumocystsis carinii or cytomegalovirus, or radiation injury.
The first symptoms to appear are dyspnea, with cough and low grade fever, commonly occurring four to ten weeks after initiation of therapy, although the time of onset of pulmonary toxicity may vary from during therapy to up to six months after the cessation of therapy.
The microscopic tissue changes due to bleomycin toxicity are frequently present as bronchiolar squamous metaplasia, reactive macrophages, atypical alveolar epithelial cells, fibrinous edema and interstitial fibrosis. The acute stage may involve capillary changes and subsequent fibrinous exudation
into alveoli producing a change similar to hyaline membrane formation and progressing to a diffuse interstitial fibrosis resembling the Hamman-Rich syndrome.
These microscopic findings are nonspecific and are similar to the changes produced in radiation pneumonitis, pneumocystic pneumonitis, and at times reaction to long standing malignant pulmonary disease.
Pulmonary function tests have revealed some alteration in the pulmonary status such as decreased total lung volume and decreased vital capacity, but these tests have proved to be of limited value in predicting pulmonary fibrosis. It has been suggested that bleomycin should be discontinued it forced vital capacity decreases rapidly.
Pulmonary toxicity is seen more commonly in smokers.
Idiosyncratic effects. Hypersensitivity reactions consisting of hypotension, lever, chills, mental confusion and wheezing have occurred in approximately 1% of patients receiving bleomycin.
This idiosyncratic reaction occurs mainly in lymphoma patients (5%), may be immediate or delayed for several hours, and usually occurs after the first or second dose. The reaction has resulted in death. Treatment of anaphylactic reactions is supportive and symptomatic and may include volume expansion, vas0pressor therapy, antihistamines and corticosteroids.
Cardiovascular. Vascular toxicities coincident with the use of bleomycin in combination with other antineoplastic agents have been reposed rarely. The events are clinically heterogeneous and may include myocardial infarction, cerebrovascular accident, thrombotic microangiopathy (hemolytic uremic syndrome) or cerebrovascular arteritis. Acute chest pain syndrome, acute
Pericarditis, fulminant fatal hyperpyrexia and fulminate, fatal angioedema have been reported.
More common reactions
Body as a whole. Fever, chills and headache frequently follow parenteral administration of bleomycin (20 to 50%). These reactions have been reported to occur most frequently with large single doses and occur within a few hours of administration lasting 4 to 12 hours. Usually, febrile reactions become less frequent with continued use of the drug but may occur sporadically and reoccur later in the treatment course.
Gastrointestinal. Anorexia, nausea and vomiting (20 to 50%) (anorexia and weight loss may persist after discontinuing therapy), tiredness.
Mucocutaneous (50%).Hypoesthesia which may progress to hyperesthesia, urticaria, erythematous swelling, tenderness, pruritus, hyperpigmentation (particularly in those areas subject to friction or pressure and in skin folds, nail cuticles, scars, and intramuscular injection sites), patchy hyperkeratosis, alopecia, ichthyosis, rash, striae, vesiculation, peeling, and bleeding, stomatitis, ulcerations of the tongue and lips. This toxicity is usually evident within one to three weeks following initiation of therapy and appears to be reversible and dose related, usually after 150,000 to 200,000 IU of bleomycin has been administered and, in general, is related to total cumulative dose. In 0.2% of patients it was necessary to discontinue treatment because of this toxicity.
When bleomycin is administered intra-arterially, dermal lesions are most common in the region supplied by the artery used. The incidence of mucocutaneous adverse events is increased when bleomycin sulfate is given in combination with radiotherapy to head and neck.
Less common reactions
Body as a whole. Idiosyncratic reactions occurring in 1% of patients (5% of lymphoma patients)
(see Severe/ life threatening reactions).
Cardiovascular. Diverse vascular toxicities (see Severe/ life-threatening reactions), hypotension (more common after intrapleural administration), sudden onset of an acute chest pain syndrome, suggestive of pleuropericarditis (although each patient must be individually evaluated, further courses of bleomycin do not appear to be contraindicated), ocular hemorrhage.
There are isolated reports of Raynaud's phenomenon occurring in patients treated with a combination of bleomycin and vinblastine with or without cisplatin or, in a few cases, with bleomycin as a single agent. It is currently unknown if the cause of the Raynaud's phenomenon in these cases is the disease, underlying vascular compromise, bleomycin, vinblastine, cisplatin induced hypomagnesaemia or a combination of any or all of these.
Central nervous system.CNS toxicity is rare, but monitoring is advised. Disorientation and aggressive behavior have been reported.
Hematological.Thrombocytopenia, leucopenia, slight depression of hemoglobin levels. Bleomycin does not frequently produce serious bone marrow toxicity.
Hepatic. Liver toxicity beginning as deterioration in liver function tests has been reported infrequently.
Injection site.Pain at injection site, phlebitis, other local reactions.
Renal. Renal toxicity beginning as deterioration in renal function tests has been reported infrequently. Hematuria and cystitis have been reported.
Respiratory. Pulmonary toxicity (10%) (see Severe/ life threatening reactions).
-
OVERDOSAGE
Symptoms: There has been no reported case of over dosage. The acute reaction would probably include hypotension, fever, rapid pulse and general symptoms of shock.
Treatment. There is no specific antidote for bleomycin Over dosage. Treatment should be symptomatic and supportive. In the everit0t respiratory complications treatment with a corticosteroid may be beneficial and the administration of a broad spectrum antibiotic is advisable.
Bleomycin is probably not dialyzable.
In case of Overdose, immediately contact the Poisons Information Centre for advice. (In Australia, call 131 126.)
-
DOSAGE AND ADMINISTRATION
Bleomycin may be given by the intramuscular, intravenous, subcutaneous or intra-arterial
routes.
Note. Because of the possibility of an anaphylactic reaction, lymphoma patients should receive test doses of between 1 and 5 units, for the first two treatments. If no acute allergic reaction occurs within four to six hours, the balance of the dose may be given. Thereafter the regular dosage schedule may be followed, if no reaction occurs.
Use in Adults
Initial treatment (Intramuscular, intravenous or subcutaneous administration). Total doses of over 300,000 IU should be given with great caution.
10,000 to 20,000 IU/m2 of body surface area given weekly or twice weekly.
Alternatively, give 15,000 IU daily for seven days followed by three weeks off treatment and repeat twice so that a total dose of approximately 300,000 IU is administered.
Improvement of lymphomas and testicular tumours is prompt, i.e. within two weeks while response by squamous cell cancers may take as long as three weeks.
A therapeutic response should be observed as the total dose approaches 150,000 IU, if this is not seen, consideration should be given to other therapy.
Note. When bleomycin is used in combination with other antineoplastic agents, pulmonary toxicities may occur at lower doses (see Precautions, Pulmonary toxicity and Interactions).
Intra-arterial administration. Intra-arterial infusion/ perfusion is employed when increased drug concentrations at the cancer site are desired. The suggested dosage schedule is 30,000 to 60,000 IU once or twice a week until the total recommended dose of 300,000 IU is reached.
Repeat treatment. In patients for whom a course of bleomycin treatment provides an initial but incomplete response, a repeat course is suggested. Patients who show superficial improvement after one course, e.g. in cases of squamous cell carcinoma, may benefit from a second course of treatment to prevent recurrence.
A repeat course may be commenced after a minimum of three to four weeks following completion of the first course, providing no sign of pulmonary toxicity has been observed (see Contraindications). A total dose of 150,000 IU for repeat treatment is recommended.
Use in Children
No information available.
Use in the elderly
Adult dose should be used with caution, particularly in patients over 70 years (see Adverse Effects, Pulmonary toxicity).
Impaired hepatic function
Use adult dose with caution.
Impaired renal function
As bleomycin is mostly excreted unchanged and as there is a high correlation between renal bleomycin clearance and creatinine clearance, impairment of function may require reduction in dosage and careful monitoring for toxicity. Dosage reductions of 40 to 75% have been recommended for patients with creatinine clearance values of less than or equal to 40 mL/minute
Reconstitution
Intramuscular, subcutaneous injection.For intramuscular or subcutaneous injection, dissolve the contents of the vial in 1 to 5 mL of sterile water for injection or sodium chloride intravenous infusion 0.9%.
Intravenous, intra-arterial injection.For intravenous or intra-arterial injection, dissolve the contents of the vial in 5 to 10 mL of diluent and administer slowly over a period of 10 minutes.
Suitable diluents are water for injections, bacteriostatic water for injection and sodium chloride intravenous infusion 0.9%. Although glucose intravenous infusion 5% has been used in the past, recent data suggests that it is not the diluent of choice, as over the concentration range of 300 to 15,000 IU/mL the content of bleomycin A2 + B2 was consistently lower when glucose intravenous infusion 5% was used.
Reconstituted solutions containing bleomycin 150 to 15,000 IU/mL prepared using the recommended diluents remain stable for periods of at least 24 hours when stored in the dark, at temperatures of 2 to 8°C. Solutions of bleomycin sulfate in sodium chloride intravenous 0.9% stored in the dark at 2 to 8°C for ten days were chemically stable. However, in order to reduce the possibility of microbiological contamination, reconstituted injections should be used as soon as practicable after preparation. If storage of the reconstituted solution is necessary, store at 2 to 8°C for no more than 24 hours. Any unused portions must be discarded in compliance with acceptable procedures for the disposal of anticancer medicines.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BLEOMYCIN
bleomycin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70121-1567 Route of Administration INTRA-ARTERIAL, INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLEOMYCIN SULFATE (UNII: 7DP3NTV15T) (BLEOMYCIN - UNII:40S1VHN69B) BLEOMYCIN 15000 [iU] Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70121-1567-1 1 in 1 CARTON; Type 0: Not a Combination Product 06/20/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 06/20/2016 Labeler - Amneal Pharmaceuticals LLC (827748190)