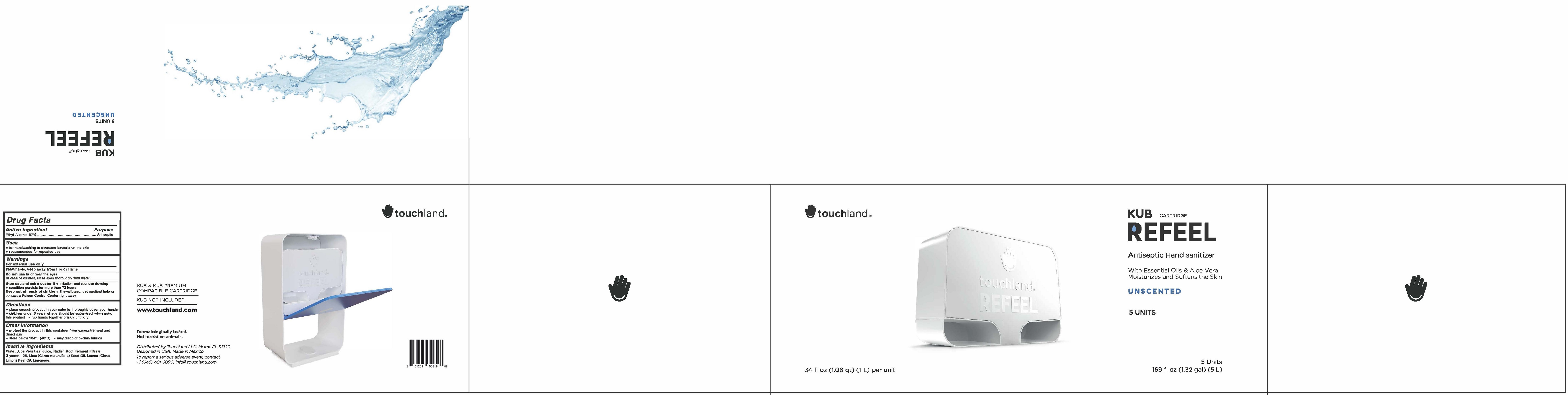
KUB CARTRIDGE REFEEL ANTISEPTIC HAND SANITIZER UNSCENTED- alcohol liquid
Touchland LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KUB Cartridge Refeel Antiseptic Hand Sanitizer Unscented
Warnings
For external use only
Flammable, keep away from fire or flame
Directions
- place enough product in your palm to thoroughly cover your hands
- children under 6 years of age should be supervised when using this product
- rub hands together briskly until dry
Other Information
- protect the product in this container from excessive heat and direct sun
- store below 104°F (40°C)
- may discolor certain fabrics
| KUB CARTRIDGE REFEEL ANTISEPTIC HAND SANITIZER UNSCENTED
alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Touchland LLC (036656461) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zobele Mexico S.A. DE C.V. | 812581940 | manufacture(72033-010) | |
Revised: 10/2021
Document Id: cf7f456c-3328-3ee9-e053-2a95a90aae45
Set id: 383345cd-0490-4610-ab42-97f810872942
Version: 3
Effective Time: 20211029
Touchland LLC