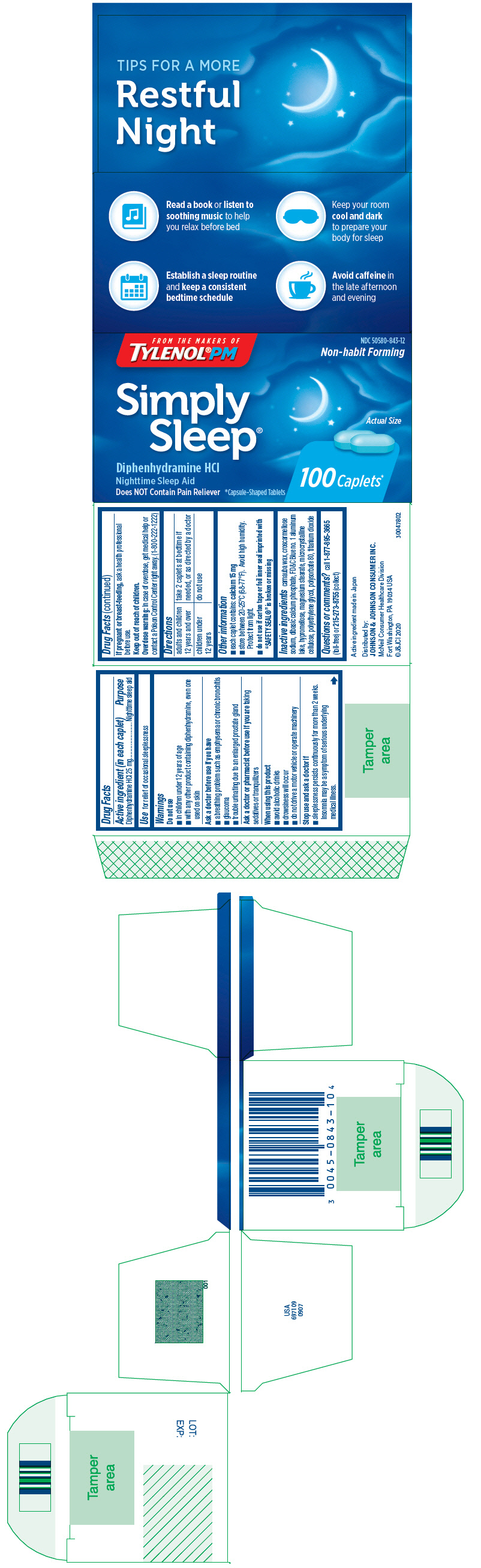
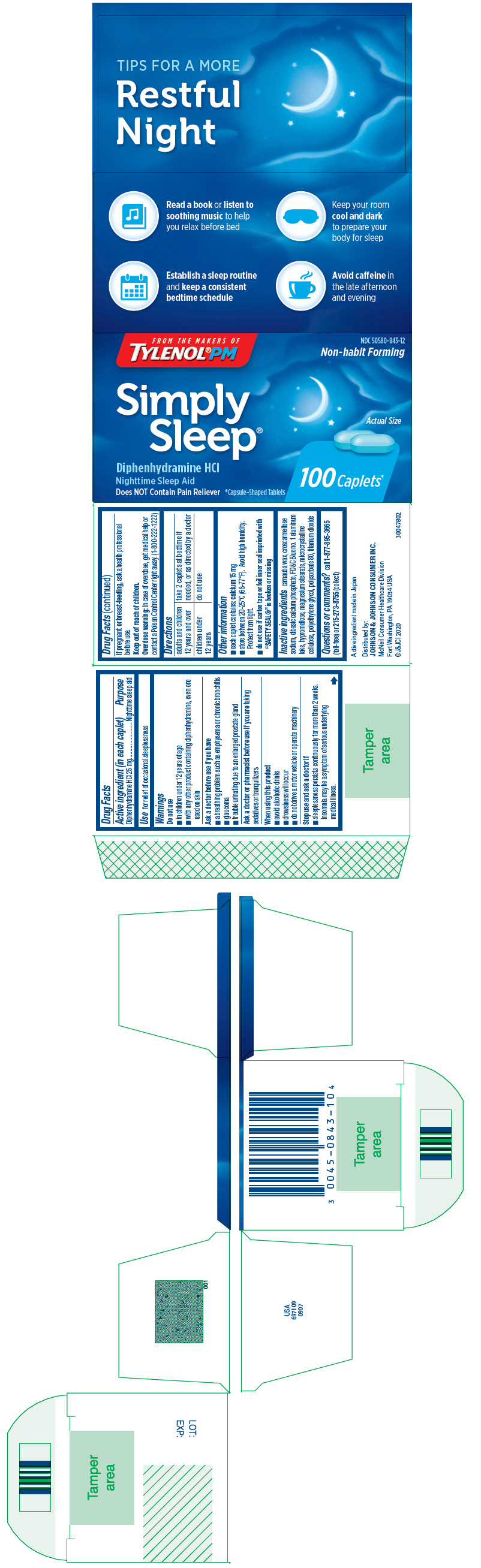
Label: SIMPLY SLEEP- diphenhydramine hydrochloride tablet, film coated
- NDC Code(s): 50580-843-11, 50580-843-12
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- avoid alcoholic drinks
- drowsiness will occur
- do not drive a motor vehicle or operate machinery
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SIMPLY SLEEP
diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-843 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue (Light blue) Score no score Shape OVAL Size 13mm Flavor Imprint Code SL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-843-11 2 in 1 CARTON 09/26/2013 12/31/2020 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:50580-843-12 1 in 1 CARTON 04/10/2013 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 02/01/2004 Labeler - Johnson & Johnson Consumer Inc. (878046358)