ARBONNE RE9 ADVANCED RESTORATIVE DAY BROAD SPECTRUM SPF 20- avobenzone, homosalate, octinoxate, and octocrylene lotion
Arbonne International, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
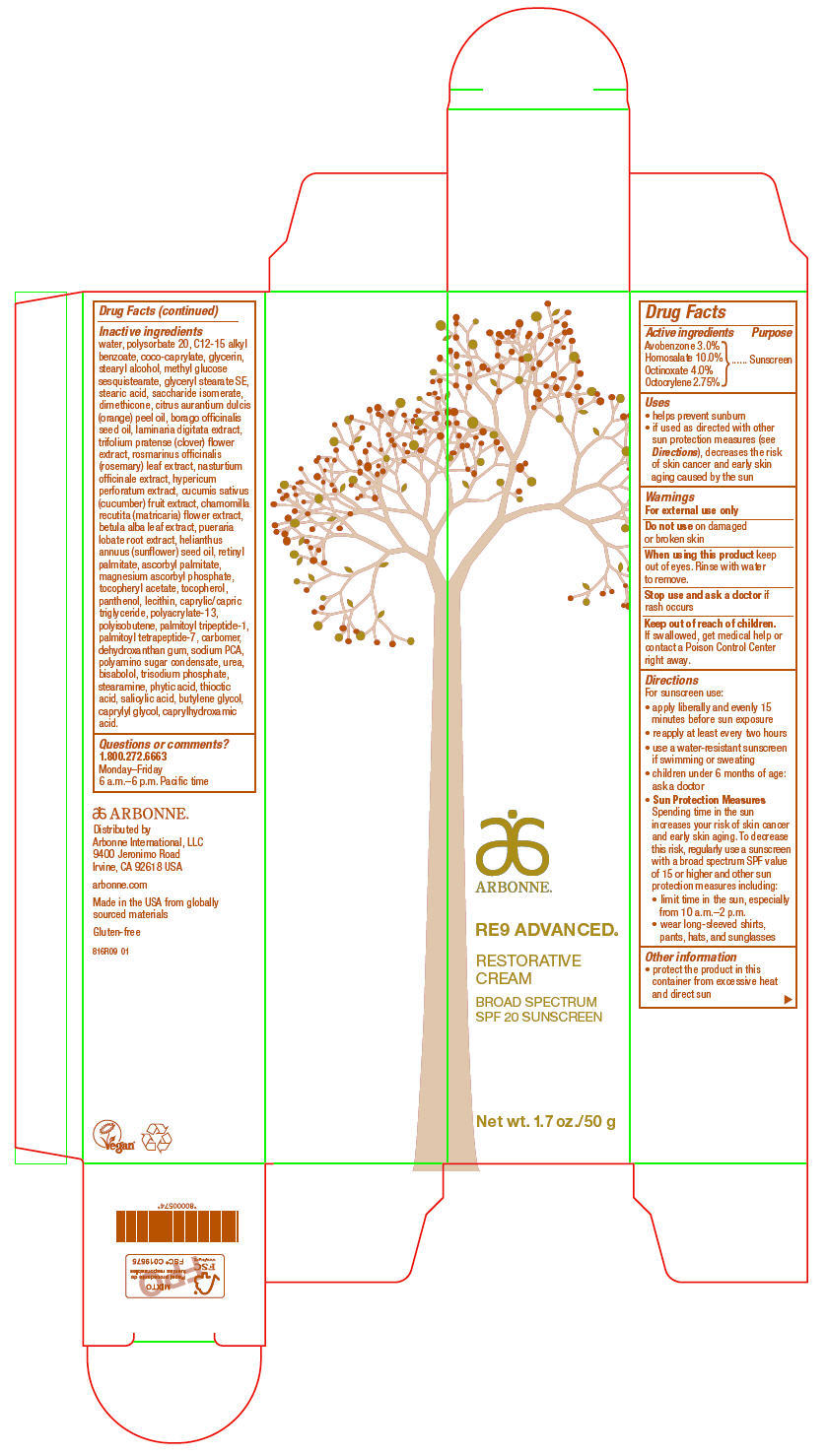
Arbonne® RE9 Advanced®
Restorative Day Broad Spectrum SPF 20
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water-resistant sunscreen if swimming or sweating
- children under 6 months of age: ask a doctor
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients
water, polysorbate 20, C12-15 alkyl benzoate, coco-caprylate, glycerin, stearyl alcohol, methyl glucose sesquistearate, glyceryl stearate SE, stearic acid, saccharide isomerate, dimethicone, citrus aurantium dulcis (orange) peel oil, borago officinalis seed oil, laminaria digitata extract, trifolium pratense (clover) flower extract, rosmarinus officinalis (rosemary) leaf extract, nasturtium officinale extract, hypericum perforatum extract, cucumis sativus (cucumber) fruit extract, chamomilla recutita (matricaria) flower extract, betula alba leaf extract, pueraria lobate root extract, helianthus annuus (sunflower) seed oil, retinyl palmitate, ascorbyl palmitate, magnesium ascorbyl phosphate, tocopheryl acetate, tocopherol, panthenol, lecithin, caprylic/capric triglyceride, polyacrylate-13, polyisobutene, palmitoyl tripeptide-1, palmitoyl tetrapeptide-7, carbomer, dehydroxanthan gum, sodium PCA, polyamino sugar condensate, urea, bisabolol, trisodium phosphate, stearamine, phytic acid, thioctic acid, salicylic acid, butylene glycol, caprylyl glycol, caprylhydroxamic acid.
| ARBONNE RE9 ADVANCED
RESTORATIVE DAY BROAD SPECTRUM SPF 20
avobenzone, homosalate, octinoxate, and octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Arbonne International, LLC (021541164) |