AVAPTA- menthol cream
Neel Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AVAPTA Penetrating Muscle and Joint
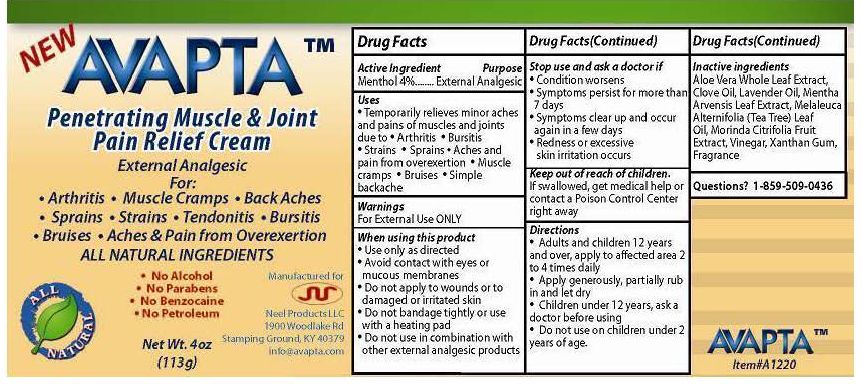
Uses
- Temporarily relieves minor aches and pains of muscles and joints due to
- Arthritis
- Bursitis
- Strains
- Sprains
- Aches and pain from overexertion
- Muscle cramps
- Bruises
- Simple backache
When using this product
- Use only as directed
- Avoid Contact with eyes or mucous membranes
- Do not apply to wounds or to damaged or irritated skin
- Do not bandage or use with a heating pad
- Do not use in combination with other external analgesic products
Stop use and ask a doctor if
- Condition worsens
- Symptoms persist for more than 7 days
- Symptoms clear up and occur again in a few days
- Redness or excessive skin irritation occurs
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control center right away
Directions
- Adults and children 12 years and over, apply to affected area 2 to 4 times daily
- Apply generously, partially rub in, penetrates and dries very quickly leaving no residue.
- Children under 12 years, ask doctor before using
- Do not use on children under 2 years of age
Inactive ingredients
Aloe Vera Whole Leaf Extract, Clove Oil, Mentha Arvensis Leaf Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Morinda Citrifolia Fruit Extract, Purified Water, Saline, Titanium dioxide, Vinegar, Xanthan Gum, Fragrance
Principal Display
AVAPTA
Penetrating Muscle and Joint
Pain Relief Cream
External Analgesic
NDC#76332-001-03
For:
- Arthritis
- Muscle Cramps
- Back Aches
- Sprains
- Strains
- Tendonitis
- Bursitis
- Bruises
- Aches and Pain from Overexertion
ALL NATURAL INGREDIENTS
- No Alcohol
- No Parabens
- No Benzocaine
- No Petroleum
Manufactured for
Neel Products LLC
1900 Woodlake Rd
Stamping Ground, KY 40379
info@avapta.com
| AVAPTA
menthol cream |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Neel Products LLC (968478102) |