Label: PROVON ANTIMICROBIAL FOAM HANDWASH- chlorhexidine gluconate 2% solution
- NDC Code(s): 21749-850-89, 21749-850-90
- Packager: Gojo Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 19, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
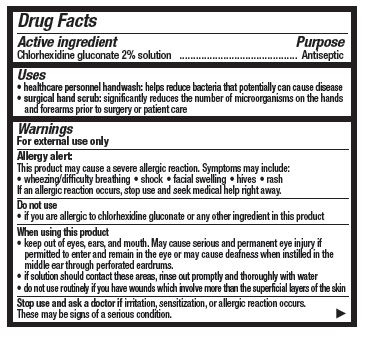
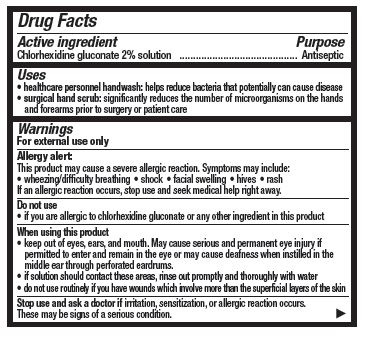
- Active ingredient
- Purpose
- Uses
- Warnings
-
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routineely if you have wounds which involve more than the superficial layers of the skin
-
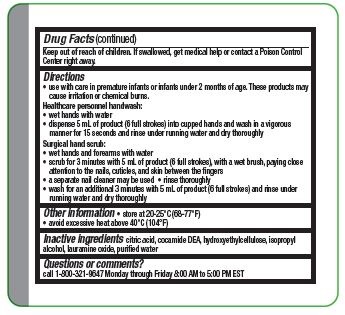
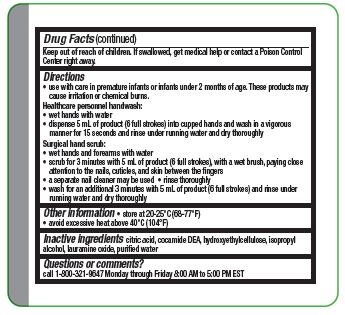
Directions
- Use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms with water
- scrub for 3 minutes with 5 ml of product (6 full strokes), with a wet brush, paying close attention to the nails, cuticles, and skin between the fingers
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product (6 full strokes) and rinse under running water and dry thoroughly
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
PROVON® Antimicrobial Foam Handwash
Chlorhexidine Gluconate 2% Solution - Antiseptic
GOJOADXFLBLC
8842-643-F

PROVON® Antimicrobial Foam Handwash
Chlorhexidine Gluconate 2% Solution - Antiseptic
GOJOLTXFLBLC
1922-643-F

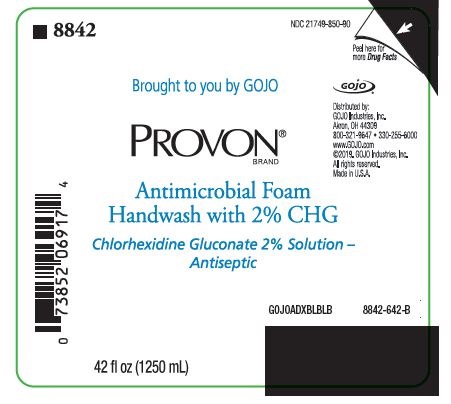
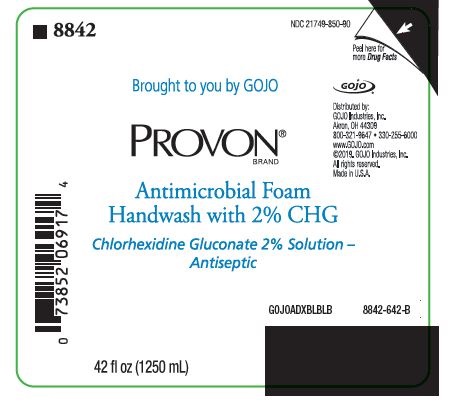
PACKAGE LABEL. SECONDARY DISPLAY PANEL
NDC 21749-850-90
Brought to you by GOJO
PROVON® BRAND
Antimicrobial Foam Handwash with 2% CHG
Chlorhexidine Gluconate 2% Solution - Antiseptic
Distributed by:
GOJO Industries, Inc.
Akron, OH 44309
800-321-9647 • 330-255-6000
www.GOJO.com
©2019. GOJO Industries, Inc.
All rights reserved.
Made in U.S.A.
GOJOADXBLBLB
8842-642-B
42 fl oz (1250 mL)

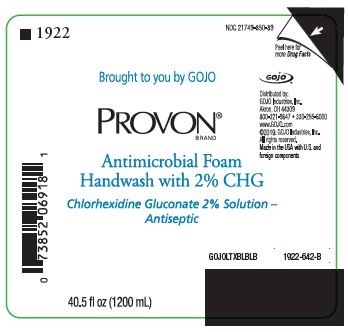
NDC 21749-850-89
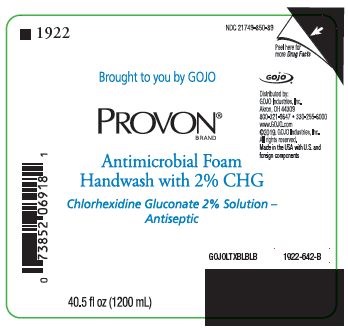
Brought to you by GOJO
PROVON® BRAND
Antimicrobial Foam Handwash with 2% CHG
Chlorhexidine Gluconate 2% Solution - Antiseptic
Distributed by:
GOJO Industries, Inc.
Akron, OH 44309
800-321-9647 • 330-255-6000
www.GOJO.com
©2019. GOJO Industries, Inc.
All rights reserved.
Made in the USA with U.S. and foreign components
GOJOLTXBLBLB
1922-642-B
40.5 fl oz (1200 mL)

-
INGREDIENTS AND APPEARANCE
PROVON ANTIMICROBIAL FOAM HANDWASH
chlorhexidine gluconate 2% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO DIETHANOLAMIDE (UNII: 92005F972D) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-850-89 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 07/01/2016 2 NDC:21749-850-90 1250 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 07/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019422 07/01/2016 Labeler - Gojo Industries, Inc. (004162038) Registrant - Xttrium Laboratories, Inc. (007470579)