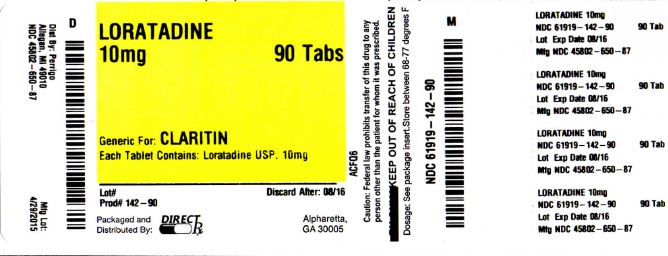
Label: LORATADINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 61919-142-10, 61919-142-30, 61919-142-90 - Packager: DIRECT RX
- This is a repackaged label.
- Source NDC Code(s): 45802-650
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 3, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- USES
- ACTIVE INGREDIENT IN EACH TABLET
- PURPOSE
-
WARNINGS
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
-
DOSAGE & ADMINISTRATION
adults and children 6 years and over1 tablet daily; not more than 1 tablet in 24 hours
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
Other information
- do not use if printed foil under cap is broken or missing
- store at 20°-25°C (68°-77°F)
Questions or comments?
1-800-719-9260
- INACTIVE INGREDIENTS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61919-142(NDC:45802-650) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score no score Shape OVAL Size 8mm Flavor Imprint Code L612 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-142-10 10 in 1 BOTTLE 1 NDC:61919-142-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:61919-142-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076301 01/01/2014 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 repack(61919-142) , relabel(61919-142)