LEXUSS 210- codeine phosphate and chlorpheniramine maleate liquid
Centurion Labs
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Lexuss 210 Liquid
CV
Active Ingredients
Each 5 mL (1 teaspoonful) of vanilla cream flavored liquid for oral administration contains:
10 mg Codeine Phosphate
2 mg Chlorpheniramine Maleate
Purpose
- Cough Suppressant
- Antihistamine
Codeine is one of the naturally occurring phenanthrene alkaloids of opium derived from the opium poppy, it is classified pharmacologically as a narcotic analgesic. Codeine phosphate may be chemically designated as 7.8-Didehydro-4, 5α-epoxy-3-methoxy-17-methylmorphinan-6-α-ol phosphate (1:1)(salt)hemihydrate. The phosphate salt of codeine occurs as white, needle-shaped crystals or white crystalline powder. Codeine phosphate is freely soluble in water and slightly soluble in alcohol and has the following chemical structure:
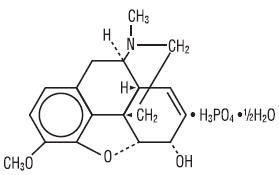
C18H21NO3 • H3PO4 • ½H2O M.W. 406.37
Chlorpheniramine Maleate is an antihistamine having the chemical name: 2-Pyridinepropanamine, γ-(4-chlorophenyl)-N,N-dimethyl-, (Z)-2-butenedioate (1:1), and has the following chemical structure:
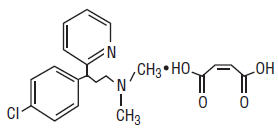
C16H19ClN2 • C4H4O4 M.W. 390.86
CLINICAL PHARMACOLOGY
Codeine
Narcotic analgesics, including codeine, exert their primary effects on the central nervous system and gastrointestinal tract. The analgesic effects of codeine are due to its central action; however, the precise sites of action have not been determined, and the mechanisms involved appear to be quite complex. Codeine resembles morphine both structurally and pharmacologically, but its actions at the doses of codeine used therapeutically are milder, with less sedation, respiratory depression, and gastrointestinal, urinary, and pupilary effects. Codeine produces an increase in biliary tract pressure, but less than morphine or meperidine. Codeine is less constipating than morphine. Codeine has good antitussive activity, although less than that of morphine at equal doses. It is used in preference to morphine, because side effects are infrequent at the usual antitussive dose of codeine. Codeine in oral therapeutic dosage does not usually exert major effects on the cardiovascular system. Narcotic analgesics may cause nausea and vomiting by stimulating the chemoreceptor trigger zone (CTZ); however, they also depress the vomiting center, so that subsequent doses are unlikely to produce vomiting. Nausea is minimal after usual oral doses of codeine. Narcotic analgesics cause histamine release, which appears to be responsible for wheals or urticaria sometimes seen at the site of injection on parenteral administration. Histamine release may also produce dilation of cutaneous blood vessels, with resultant flushing of the face and neck, pruritus, and sweating.
Codeine and its salts are well absorbed following both oral and parenteral administration. Codeine is about 2/3 as effective orally as parenterally. Codeine is metabolized primarily in the liver by enzymes of the endoplasmic reticulum, where it undergoes 0-demethylation, N-demethylation, and partial conjugation with glucuronic acid. The drug is excreted primarily in the urine, largely as inactive metabolites and small amounts of free and conjugated morphine. Negligible amounts of codeine and its metabolites are found in the feces. Following oral or subcutaneous administration of codeine, the onset of analgesia occurs within 15 to 30 minutes and lasts for four to six hours. The cough-depressing action, in animal studies, was observed to occur 15 minutes after oral administration of codeine, peak action at 45 to 60 minutes after ingestion. The duration of action, which is dose-dependent, usually did not exceed 3 hours.
Chlorpheniramine
Chlorpheniramine Maleate is an alkylamine-type antihistamine that possesses anticholinergic and sedative effects. Antihistamines competitively antagonize histamine at the H1 receptor site. Thus, activation of H1 receptors by released histamine which results in increased vascular permeability, increased mucus production, pruritis and sneezing is prevented.
INDICATIONS
Temporarily relieves runny nose and alleviates sneezing, itching of the nose or throat, and itchy watery eyes due to hay fever or allergic rhinitis; for the temporary control of cough due to minor throat and bronchial irritation associated with the common cold or inhaled irritants; calms the cough control center and relieves coughing.
CONTRAINDICATIONS
Codeine is contraindicated in patients with a known hypersensitivity to the drug. Antihistamines and codeine are both contraindicated for use in the treatment of lower respiratory tract symptoms, including asthma. Antihistamines and anticholinergics are contraindicated in patients with narrow-angle glaucoma, urinary retention, peptic ulcer and during an asthma attack. Contraindicated in breast-feeding mothers.
WARNINGS
Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor. When using this product, Lexuss 210 Liquid may cause marked drowsiness.
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache, consult a doctor.
Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm (mucus) unless directed by a physician.
Adults and children who have a chronic pulmonary disease or shortness of breath, or children who are taking other drugs, should not take this product unless directed by a physician.
Dosage of codeine SHOULD NOT BE INCREASED if cough fails to respond; an unresponsive cough should be reevaluated in 5 days or sooner for possible underlying pathology, such as foreign body or lower respiratory tract disease.
Codeine may cause or aggravate constipation.
Respiratory depression leading to arrest, coma, and death has occurred with the use of codeine antitussive in young children, particularly in the under-one-year infants whose ability to deactivate the drug is not fully developed.
Administration of codeine may be accomplished by histamine release and should be used with caution in atopic children.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotic analgesics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, intracranial lesions, or a reexisting increase in intracranial pressure. Narcotics may produce adverse reactions which may obscure the clinical course of patients with head injuries.
Asthma and Other Respiratory Conditions
Narcotic analgesics or cough suppressants, including codeine, should not be used in asthmatic patients. Nor should they be used in acute febrile illness associated with productive cough or in chronic respiratory disease where interference with ability to clear the tracheobronchial tree of secretions would have a deleterious effect on the patient's respiratory function.
Hypotensive Effect
Codeine may produce orthostatic hypotension in ambulatory patients.
Antihistamines may cause excitability especially in children. Do not take this product, unless directed by a physician, if you have a breathing problem such as emphysema or chronic bronchitis, or if you have glaucoma or difficulty in urination due to enlargement of the prostate gland. May cause drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor. Use caution when driving a motor vehicle or operating machinery.
PRECAUTIONS
General
Before prescribing medication to suppress or modify cough, it is important that the underlying cause of cough is identified, that modification of cough does not increase the risk of clinical or physiological complications, and that appropriate therapy for the primary disease is instituted.
Narcotic analgesics, including codeine, should be administered with caution and the initial dose reduced in patients with acute abdominal conditions, convulsive disorders, significant hepatic or renal impairment, fever, hypothyroidism, Addison's disease, ulcerative colitis, prostatic hypertrophy, in patients with recent gastrointestinal or urinary tract surgery, and in the very young or elderly or debilitated patients.
Ultra-rapid Metabolizers of Codeine
Some individuals may be ultra-rapid metabolizers due to a specific CYP2D6*2x2 genotype. These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regiments, individuals who are ultra-rapid metabolizers may experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing.
The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1-10% in Caucasians, 3% in African Americans, and 16-28% in North Africans, Ethiopians and Arabs. Data is not available for other ethnic groups.
When physicians prescribe codeine-containing drugs, they should choose the lowest effective dose for the shortest period of time and should inform their patients about these risks and the signs of morphine overdose.
Drug/Laboratory Test Interactions
In patients receiving MAO inhibitors, an initial small test dose is advisable to allow observation of any excessive narcotic effects or MAOI interaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No data are available on the long-term potential for carcinogenesis, mutagenesis, or impairment of fertility in animals or humans.
Pregnancy
Pregnancy Category C
A study in rats and rabbits reported no teratogenic effect of codeine administered during the period of organogenesis in doses ranging from 5 to 120 mg/kg. In the rat, doses at the 120-mg/kg level, in the toxic range for the adult animal were associated with an increase in embryo resorption at the time of implantation. In another study a single 100-mg/kg dose of codeine administered to pregnant mice reportedly resulted in delayed ossification in the offspring. There are no studies in humans, and the significance of these findings to humans, if any, is not known. Do not use during third trimester.
Labor and Delivery
Narcotic analgesics cross the placental barrier. The closer to delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Narcotic analgesics should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required.
Nursing Mothers
Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose-dependent. Despite the common use of codeine products to manage postpartum pain, reports of adverse events in infants are rare. However, some women are ultra-rapid metabolizers of codeine. These women achieve higher-than-expected serum levels of codeine's active metabolite, morphine, leading to higher-than-expected levels of morphine in breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, maternal use of codeine can potentially lead to serious adverse reactions, including death, in nursing infants.
The risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and baby. Caution should be exercised when codeine is administered to a nursing woman. If a codeine containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Mothers using codeine should be informed about when to seek immediate medical care and how to identify the signs and symptoms of neonatal toxicity, such as drowsiness or sedation, difficulty breastfeeding, breathing difficulties, and decreased tone in their baby. Nursing mothers who are ultra-rapid metabolizers may also experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. Prescribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine during breastfeeding.
Information for Patients
Codeine may cause marked drowsiness or may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. Ambulatory patients should be told to avoid engaging in such activities until it is known that they do not become drowsy or dizzy from codeine therapy.
The concomitant use of alcohol or other central nervous system depressants, including narcotic analgesics, sedatives, hypnotics, and tranquilizers, may have an additive effect and should be avoided or their dosage reduced.
Patients should be advised to report any involuntary muscle movements. Avoid prolonged exposure to the sun. Codeine, like other narcotic analgesics, may produce orthostatic hypotension in some ambulatory patients. Patients should be cautioned accordingly. Caution patients that some people have a variation in a liver enzyme and change codeine into morphine more rapidly and completely than other people. These people are ultra-rapid metabolizers and are more likely to have higher-than-normal levels of morphine in their blood after taking codeine which can result in overdose symptoms such as extreme sleepiness, confusion, or shallow breathing. In most cases, it is unknown if someone is an ultra-rapid codeine metabolizer. Nursing mothers taking codeine can also have higher morphine levels in their breast milk if they are ultra-rapid metabolizers. These higher levels of morphine in breast milk may lead to life-threatening or fatal side effects in nursing babies. Instruct nursing mothers to watch for signs of morphine toxicity in their infants including increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to the baby's doctor immediately if they notice these signs and, if they cannot reach the doctor right away, to take the baby to an emergency room or call 911 (or local emergency services).
Caution patient using Chlorpheniramine that each product has specific dosing instructions and to read package label before using and not to exceed dose or frequency of administration instructions. Advise patient to take each dose without regard to meals, but to take with food if stomach upset occurs. Advise patient or caregiver using oral syrup to measure and administer prescribed dose using dosing syringe, dosing spoon, or dosing cup. Advise patient that if a dose is missed, to take it as soon as possible unless it is nearing time for the next scheduled dose. If it is nearing time for next scheduled dose, advise patient to skip the missed dose and take the next dose at the regularly scheduled time. Caution patient not to double the dose to catch up. Advise patient that if allergy symptoms are not controlled, not to increase the dose of medication or frequency of use but to inform health care provider. Caution patient that larger doses or more frequent dosing does not increase efficacy and may cause excessive drowsiness or other adverse reactions. Instruct patient to stop taking drug and immediately report any of these symptoms to health care provider: persistent dizziness; excessive drowsiness; severe dry mouth, nose, or throat; flushing; unexplained shortness of breath or difficulty breathing; unusual tiredness or weakness; sore throat, fever, or other signs of infection; bleeding or unusual bruising; fast or irregular heartbeat; excitability, confusion, or changes in thinking or behavior; chest tightness; difficulty with urination. Advise patient medication may cause drowsiness or dizziness and not to drive or perform other activities requiring mental alertness until tolerance is determined. Advise patient to take sips of water, suck on ice chips or sugarless hard candy, or chew sugarless gum if dry mouth occurs. Caution patient alcohol and other CNS depressants (e.g., sedatives) will have additional sedative effects if taken with Chlorpheniramine. Caution patient not to take any other OTC antihistamines while taking this medication unless advised by health care provider. Caution patient that medication may cause sensitivity to sunlight and to avoid excessive exposure to the sun or UV light (e.g., tanning booths) and to wear protective clothing and use sunscreens until tolerance is determined. If patient is to have allergy skin testing, advise patient not to take the medication for at least 4 days before the skin testing.
ADVERSE REACTIONS
Phenylephrine HCl
Codeine
Nervous System - CNS depression, particularly respiratory depression, and to a lesser extent circulatory depression; light-headedness, dizziness, sedation, euphoria, dysphoria, headache, transient hallucination, disorientation, visual disturbances, and convulsions.
Cardiovascular – Tachycardia, bradycardia, palpitation, faintness, syncope, orthostatic hypotension (common to narcotic analgesics).
Gastrointestinal – Nausea, vomiting, constipation, and biliary tract spasm. Patients with chronic ulcerative colitis may experience increased colonic motility; in patients with acute ulcerative colitis, toxic dilation has been reported.
Genitourinary – Oliguria, urinary retention, antidiuretic effect has been reported (common to narcotic analgesics).
Allergic – Infrequent pruritus, giant urticaria, angioneurotic edema, and laryngeal edema.
Other – Flushing of the face, sweating and pruritus (due to opiate-induced histamine release); weakness.
DRUG ABUSE AND DEPENDENCE
Lexuss 210 Liquid is subject to Federal Controlled Substances Act (Schedule V).
Abuse
Codeine is known to be subject to abuse; however, the abuse potential of oral codeine appears to be quite low. Even parenteral codeine does not appear to offer the psychic effects sought by addicts to the same degree as heroin or morphine. However, codeine must be administered only under close supervision to patients with a history of drug abuse or dependence.
OVERDOSAGE
Serious overdose with codeine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. The triad of coma, pinpoint pupils, and respiratory depression is strongly suggestive of opiate poisoning. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur. It is difficult to determine what constitutes a standard toxic or lethal dose. However, the lethal oral dose of codeine in an adult is reported to be in the range of 0.5 to 1 gram. Infants and children are believed to be relatively more sensitive to opiates on a body-weight basis. Elderly patients are also comparatively intolerant to opiates. Treatment of overdosage with codeine is essentially symptomatic and supportive. Only in cases of extreme overdosage or individual sensitivity do vital signs including respiration, pulse, blood pressure, temperature, and EKG need to be monitored. Activated charcoal orally or by lavage may be given, or sodium or magnesium sulfate orally as a cathartic. Attention should be given to the reestablishment of adequate respiratory exchange through provision of a patient airway and institution of assisted or controlled ventilation. The narcotic antagonist, naloxone hydrochloride, may be administered when significant respiratory depression occurs with codeine; Severe hypotension usually responds to the administration of norepinephrine or Phenylephrine. EPINEPHRINE SHOULD NOT BE USED, since its use in a patient with partial adrenergic blockade may further lower the blood pressure. Limited experience with dialysis indicates that it is not helpful. Overdosage with Chlorpheniramine may cause hallucinations, convulsions, and death. Antihistamines may diminish mental alertness. In young children, they may produce paradoxical excitation.
DOSAGE AND ADMINISTRATION1
Adults and children 12 years of age and over: 1 to 2 teaspoonfuls (5-10 mL) every 4 to 6 hours. Not to exceed 8 teaspoonfuls in a 24 hour period.
Children 6 to under 12 years of age: 1/2 to 1 teaspoonful (2.5 - 5 mL) every 4 to 6 hours. Not to exceed 4 teaspoonfuls in a 24 hour period.
Also contains the following inactive ingredients (in alphabetical order): Citric Acid, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sorbitol Solution 70%, Sucralose, Vanilla Ice Cream Flavor
- 1
- In mild cases or in particularly sensitive patients, less frequent or reduced doses may be appropriate and adequate. Shake liquid well before use.
HOW SUPPLIED
Lexuss 210 Liquid is a clear solution with a vanilla aroma which contains 10 mg Codeine Phosphate and 2 mg Chlorpheniramine Maleate, available in 16 fl oz (473 mL) bottles.
NDC 23359-016-16
| LEXUSS 210
codeine phosphate and chlorpheniramine maleate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Centurion Labs (016481957) |
