CREST PRO-HEALTH FOR LIFE- cetylpyridinium chloride rinse
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crest
® Pro-Health™
For Life
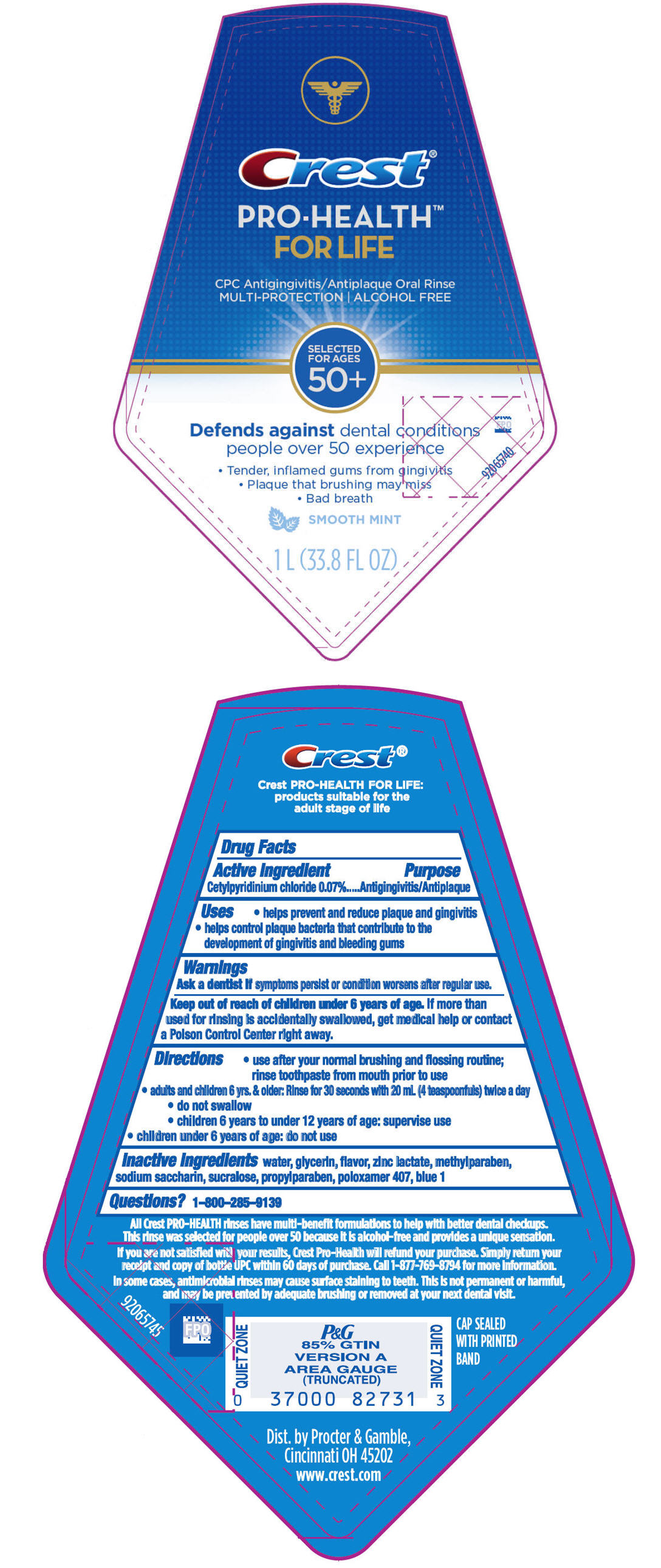
Uses
- helps prevent and reduce plaque and gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
Directions
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
Inactive ingredients
water, glycerin, flavor, zinc lactate, methylparaben, sodium saccharin, sucralose, propylparaben, poloxamer 407, blue 1
PRINCIPAL DISPLAY PANEL - 1 L Bottle Label
Crest®
PRO-HEALTH™
FOR LIFE
CPC Antigingivitis/Antiplaque Oral Rinse
MULTI-PROTECTION | ALCOHOL FREE
SELECTED
FOR AGES
50+
Defends against dental conditions
people over 50 experience
- Tender, inflamed gums from gingivitis
- Plaque that brushing may miss
- Bad breath
SMOOTH MINT
1 L (33.8 FL OZ)
92065740
| CREST PRO-HEALTH
FOR LIFE
cetylpyridinium chloride rinse |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |