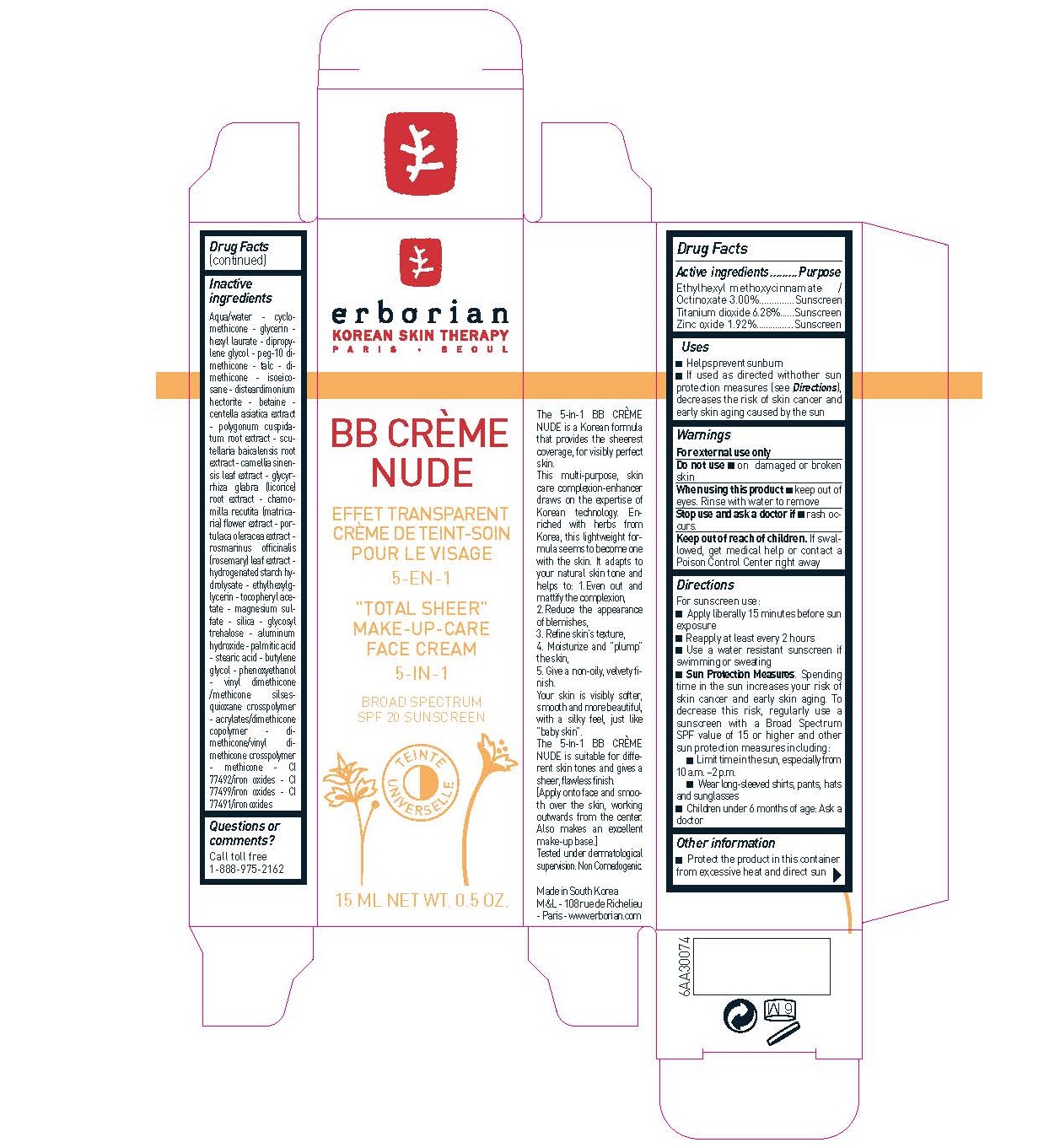
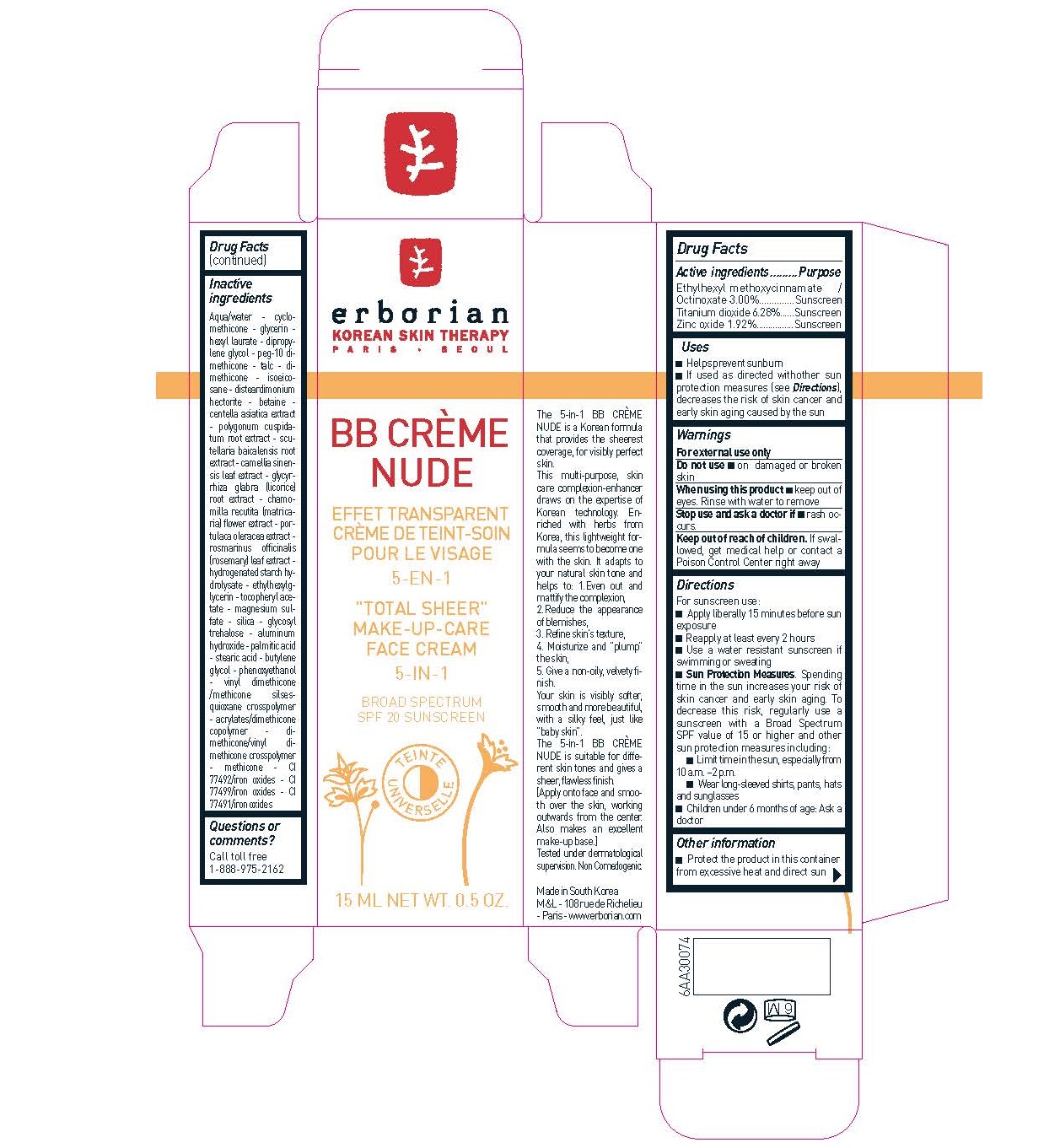
Label: ERBORIAN - BB CREME NUDE SPF20- octinoxate, titanium dioxide, zinc oxide cream
- NDC Code(s): 10345-902-15, 10345-902-45
- Packager: LABORATOIRES M&L
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use: -Apply liberally 15 minutes before sun exposure -Reapply at least every 2 hours -Use a water resistant sunscreen if swimming or sweating - . Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: -Limit time in the sun, especially from 10 a.m. –2 p.m. -Wear long-sleeved shirts, pants, hats and sunglasses -Children under 6 months of age: Ask a doctor
Sun Protection Measures -
Inactive Ingredients
Aqua/water - cyclomethicone- glycerin hexyl laurate - dipropylene glycol - peg-10 dimethicone- talc - dimethicone- isoeicosane-disteardimonium hectorite - betaine -centella asiatica extract- polygonum cuspidatum root extract - scutellaria baicalensis root extract - camellia sinensis leaf extract - glycyrrhiza glabra (licorice) root extract - chamomilla recutita (matricaria) flower extract - portulaca oleracea extract - rosmarinus officinalis (rosemary) leaf extract - hydrogenated starch hydrolysate - ethylhexylglycerin - tocopheryl acetate - magnesium sulfate - silica - glycosyl trehalose - aluminum hydroxide - palmitic acid - stearic acid - butylene glycol - phenoxyethanol - vinyl dimethicone/methicone silsesquioxane crosspolymer - acrylates/dimethicone copolymer - dimethicone/vinyl dimethicone crosspolymer -methicone - CI 77492/iron oxides - CI 77499/iron oxides - CI 77491/iron oxides
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ERBORIAN - BB CREME NUDE SPF20
octinoxate, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10345-902 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 30 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 62.8 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 19.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE (UNII: NMQ347994Z) DIPROPYLENE GLYCOL (UNII: E107L85C40) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) ISOEICOSANE (UNII: AR294KAG3T) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) BETAINE (UNII: 3SCV180C9W) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HYDROGENATED STARCH HYDROLYSATE (UNII: 27F77DSJ5V) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10345-902-45 1 in 1 BOX 08/17/2016 1 45 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10345-902-15 1 in 1 BOX 08/17/2016 2 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/17/2016 Labeler - LABORATOIRES M&L (262533623)