Label: TRIZIVIR- abacavir sulfate, lamivudine, and zidovudine tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 53808-0990-1 - Packager: State of Florida DOH Central Pharmacy
- This is a repackaged label.
- Source NDC Code(s): 49702-217
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 24, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRIZIVIR safely and effectively. See full prescribing information for TRIZIVIR.
TRIZIVIR (abacavir sulfate, lamivudine, and zidovudine) Tablets, for oral use
Initial U.S. Approval: 2000WARNING: RISK OF HYPERSENSITIVITY REACTIONS, HEMATOLOGIC TOXICITY, MYOPATHY, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY, EXACERBATIONS OF HEPATITIS B
See full prescribing information for complete boxed warning.
- •
- Serious and sometimes fatal hypersensitivity reactions have been associated with abacavir-containing products. (5.1)
- •
- Hypersensitivity to abacavir is a multi-organ clinical syndrome. (5.1)
- •
- Patients who carry the HLA-B*5701 allele are at high risk for experiencing a hypersensitivity reaction to abacavir. (5.1)
- •
- Discontinue TRIZIVIR as soon as a hypersensitivity reaction is suspected. Regardless of HLA-B*5701 status, permanently discontinue TRIZIVIR if hypersensitivity cannot be ruled out. (5.1)
- •
- Following a hypersensitivity reaction to abacavir, NEVER restart TRIZIVIR or any other abacavir-containing product. (5.1)
- •
- Hematologic toxicity, including neutropenia and anemia, has been associated with the use of zidovudine, a component of TRIZIVIR. (5.2)
- •
- Symptomatic myopathy associated with prolonged use of zidovudine. (5.3)
- •
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. (5.4)
- •
- Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and human immunodeficiency virus (HIV-1) and have discontinued lamivudine, a component of TRIZIVIR. Monitor hepatic function closely in these patients and, if appropriate, initiate anti-hepatitis B treatment. (5.5)
INDICATIONS AND USAGE
TRIZIVIR, a combination of abacavir, lamivudine, and zidovudine, each nucleoside analogue HIV-1 reverse transcriptase inhibitors, is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. (1) (1)
DOSAGE AND ADMINISTRATION
- •
- A medication guide and warning card should be dispensed with each new prescription and refill. (2)
- •
- Adults and Adolescents: 1 tablet twice daily. (2.1)
- •
- Not recommended in adolescents who weigh less than 40 kg. (2.1)
- •
- Do not prescribe for patients requiring dosage adjustment or patients with hepatic impairment. (2.2)
DOSAGE FORMS AND STRENGTHS
Tablets contain 300 mg abacavir, 150 mg of lamivudine, and 300 mg of zidovudine. (3) (3)
CONTRAINDICATIONS
- •
- Previously demonstrated hypersensitivity to abacavir or any other component of the product. (4, 5.1, 6)
- •
- Hepatic impairment. (4)
WARNINGS AND PRECAUTIONS
- •
- See boxed warning for information about the following: hypersensitivity reactions, hematologic toxicity, myopathy, lactic acidosis and severe hepatomegaly, and severe acute exacerbations of hepatitis B. (5.1, 5.2, 5.3, 5.4, 5.5)
- •
- Hepatic decompensation, some fatal, has occurred in HIV-1/HCV co-infected patients receiving combination antiretroviral therapy and interferon alfa with or without ribavirin. Discontinue TRIZIVIR as medically appropriate and consider dose reduction or discontinuation of interferon alfa, ribavirin, or both. (5.6)
- •
- Exacerbation of anemia has been reported in HIV-1/HCV co-infected patients receiving ribavirin and zidovudine. Coadministration of ribavirin and zidovudine is not advised. (5.6)
- •
- Immune reconstitution syndrome (5.7) and redistribution/accumulation of body fat (5.8) have been reported in patients treated with combination antiretroviral therapy.
- •
- TRIZIVIR should not be administered with other products containing abacavir, lamivudine, or zidovudine; or with emtricitabine. (5.11)
ADVERSE REACTIONS
The most commonly reported adverse reactions (incidence ≥10%) in clinical trials were nausea, headache, malaise and fatigue, and nausea and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ViiV Healthcare at 1-877-844-8872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Concomitant use with the following drugs should be avoided: stavudine (7.1), doxorubicin (7.2).
- •
- Ethanol: Decreases the elimination of abacavir. (7.3)
- •
- Bone marrow suppressive/cytotoxic agents: May increase the hematologic toxicity of zidovudine. (7.4)
- •
- Methadone: An increased methadone dose may be required in a small number of patients. (7.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF HYPERSENSITIVITY REACTIONS, HEMATOLOGIC TOXICITY, MYOPATHY, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY, EXACERBATIONS OF HEPATITIS B
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults and Adolescent Patients
2.2 Dosage Adjustment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5.1 Hypersensitivity Reaction
5.2 Hematologic Toxicity/Bone Marrow Suppression
5.3 Myopathy
5.4 Lactic Acidosis/Hepatomegaly With Steatosis
5.5 Patients With HIV-1 and Hepatitis B Virus Co-infection
5.6 Use With Interferon- and Ribavirin-Based Regimens
5.7 Immune Reconstitution Syndrome
5.8 Fat Redistribution
5.9 Myocardial Infarction
5.10 Therapy Experienced Patients
5.11 Use With Other Abacavir-, Lamivudine-, Zidovudine-, and/or Emtricitabine-Containing Products
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antiretroviral Agents
7.2 Doxorubicin
7.3 Ethanol
7.4 Hematologic/Bone Marrow Suppressive/Cytotoxic Agents
7.5 Interferon- and Ribavirin-Based Regimens
7.6 Methadone
7.7 Trimethoprim/Sulfamethoxazole (TMP/SMX)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients With Impaired Renal Function
8.7 Patients With Impaired Hepatic Function
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF HYPERSENSITIVITY REACTIONS, HEMATOLOGIC TOXICITY, MYOPATHY, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY, EXACERBATIONS OF HEPATITIS B
Hypersensitivity Reactions: Serious and sometimes fatal hypersensitivity reactions have been associated with abacavir sulfate, a component of TRIZIVIR. Hypersensitivity to abacavir is a multi-organ clinical syndrome usually characterized by a sign or symptom in 2 or more of the following groups: (1) fever, (2) rash, (3) gastrointestinal (including nausea, vomiting, diarrhea, or abdominal pain), (4) constitutional (including generalized malaise, fatigue, or achiness), and (5) respiratory (including dyspnea, cough, or pharyngitis). Discontinue TRIZIVIR as soon as a hypersensitivity reaction is suspected.
Patients who carry the HLA-B*5701 allele are at high risk for experiencing a hypersensitivity reaction to abacavir. Prior to initiating therapy with abacavir, screening for the HLA-B*5701 allele is recommended; this approach has been found to decrease the risk of hypersensitivity reaction. Screening is also recommended prior to reinitiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir. HLA-B*5701-negative patients may develop a suspected hypersensitivity reaction to abacavir; however, this occurs significantly less frequently than in HLA-B*5701-positive patients.
Regardless of HLA-B*5701 status, permanently discontinue TRIZIVIR if hypersensitivity cannot be ruled out, even when other diagnoses are possible.
Following a hypersensitivity reaction to abacavir, NEVER restart TRIZIVIR or any other abacavir-containing product because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
Reintroduction of TRIZIVIR or any other abacavir-containing product, even in patients who have no identified history or unrecognized symptoms of hypersensitivity to abacavir therapy, can result in serious or fatal hypersensitivity reactions. Such reactions can occur within hours [see Warnings and Precautions (5.1)].
Hematologic Toxicity: Zidovudine, a component of TRIZIVIR, has been associated with hematologic toxicity, including neutropenia and severe anemia, particularly in patients with advanced Human Immunodeficiency Virus (HIV-1) disease [see Warnings and Precautions (5.2)].
Myopathy: Prolonged use of zidovudine has been associated with symptomatic myopathy [see Warnings and Precautions (5.3)].
Lactic Acidosis and Severe Hepatomegaly: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including abacavir, lamivudine, zidovudine, and other antiretrovirals [see Warnings and Precautions (5.4)].
Exacerbations of Hepatitis B: Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and HIV-1 and have discontinued lamivudine, which is one component of TRIZIVIR. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue TRIZIVIR and are co-infected with HIV-1 and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.5)].
-
1 INDICATIONS AND USAGE
TRIZIVIR is indicated in combination with other antiretrovirals or alone for the treatment of HIV-1 infection.
Additional important information on the use of TRIZIVIR for treatment of HIV-1 infection:
- •
- TRIZIVIR is one of multiple products containing abacavir. Before starting TRIZIVIR, review medical history for prior exposure to any abacavir-containing product in order to avoid reintroduction in a patient with a history of hypersensitivity to abacavir [see Warnings and Precautions (5.1), Adverse Reactions (6)].
- •
- TRIZIVIR is a fixed-dose combination of 3 nucleoside analogues: abacavir, lamivudine, and zidovudine and is intended only for patients whose regimen would otherwise include these 3 components.
- •
- Limited data exist on the use of TRIZIVIR alone in patients with higher baseline viral load levels (>100,000 copies/mL) [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
- •
- A Medication Guide and Warning Card that provide information about recognition of hypersensitivity reactions should be dispensed with each new prescription and refill.
- •
- TRIZIVIR can be taken with or without food.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TRIZIVIR Tablets are contraindicated in patients with:
- •
- previously demonstrated hypersensitivity to abacavir or any other component of the product. NEVER restart TRIZIVIR or any other abacavir-containing product following a hypersensitivity reaction to abacavir, regardless of HLA-B*5701 status [see Warnings and Precautions (5.1), Adverse Reactions (6)].
- •
- hepatic impairment [see Use in Specific Populations (8.7)].
-
WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reaction
Serious and sometimes fatal hypersensitivity reactions have been associated with TRIZIVIR and other abacavir-containing products. Patients who carry the HLA-B*5701 allele are at high risk for experiencing a hypersensitivity reaction to abacavir. Prior to initiating therapy with abacavir, screening for the HLA-B*5701 allele is recommended; this approach has been found to decrease the risk of a hypersensitivity reaction. Screening is also recommended prior to reinitiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir. For HLA-B*5701-positive patients, treatment with an abacavir-containing regimen is not recommended and should be considered only with close medical supervision and under exceptional circumstances when the potential benefit outweighs the risk.
HLA-B*5701-negative patients may develop a hypersensitivity reaction to abacavir; however, this occurs significantly less frequently than in HLA-B*5701-positive patients. Regardless of HLA-B*5701 status, permanently discontinue TRIZIVIR if hypersensitivity cannot be ruled out, even when other diagnoses are possible.
Important information on signs and symptoms of hypersensitivity, as well as clinical management, is presented below.
Signs and Symptoms of Hypersensitivity: Hypersensitivity to abacavir is a multi-organ clinical syndrome usually characterized by a sign or symptom in 2 or more of the following groups.
Group 1: Fever
Group 2: Rash
Group 3: Gastrointestinal (including nausea, vomiting, diarrhea, or abdominal pain)
Group 4: Constitutional (including generalized malaise, fatigue, or achiness)
Group 5: Respiratory (including dyspnea, cough, or pharyngitis)
Hypersensitivity to abacavir following the presentation of a single sign or symptom has been reported infrequently.
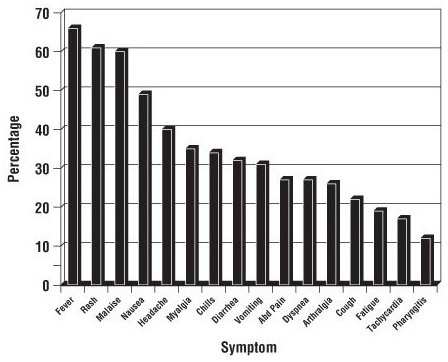
Hypersensitivity to abacavir was reported in approximately 8% of 2,670 subjects (n = 206) in 9 clinical trials (range: 2% to 9%) with enrollment from November 1999 to February 2002. Data on time to onset and symptoms of suspected hypersensitivity were collected on a detailed data collection module. The frequencies of symptoms are shown in Figure 1. Symptoms usually appeared within the first 6 weeks of treatment with abacavir, although the reaction may occur at any time during therapy. Median time to onset was 9 days; 89% appeared within the first 6 weeks; 95% of subjects reported symptoms from 2 or more of the 5 groups listed above.
A trial with ZIAGEN® (abacavir sulfate) used double-blind ascertainment of suspected hypersensitivity reactions. During the blinded portion of the trial, suspected hypersensitivity to abacavir was reported by investigators in 9% of 324 subjects in the abacavir group and 3% of 325 subjects in the zidovudine group.
Figure 1. Hypersensitivity-Related Symptoms Reported With ≥10% Frequency in Clinical Trials (n = 206 Subjects)
Other less common signs and symptoms of hypersensitivity include lethargy, myolysis, edema, abnormal chest x-ray findings (predominantly infiltrates, which can be localized), and paresthesia. Anaphylaxis, liver failure, renal failure, hypotension, adult respiratory distress syndrome, respiratory failure, and death have occurred in association with hypersensitivity reactions.
Physical findings associated with hypersensitivity to abacavir in some subjects include lymphadenopathy, mucous membrane lesions (conjunctivitis and mouth ulcerations), and rash. The rash usually appears maculopapular or urticarial, but may be variable in appearance. There have been reports of erythema multiforme. Hypersensitivity reactions have occurred without rash.
Laboratory abnormalities associated with hypersensitivity to abacavir in some subjects include elevated liver function tests, elevated creatinine phosphokinase, elevated creatinine, and lymphopenia.
Clinical Management of Hypersensitivity: Discontinue TRIZIVIR as soon as a hypersensitivity reaction is suspected. To minimize the risk of a life-threatening hypersensitivity reaction, permanently discontinue TRIZIVIR if hypersensitivity cannot be ruled out, even when other diagnoses are possible (e.g., acute onset respiratory diseases such as pneumonia, bronchitis, pharyngitis, or influenza; gastroenteritis; or reactions to other medications).
Following a hypersensitivity reaction to abacavir, NEVER restart TRIZIVIR or any other abacavir-containing product because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
When therapy with TRIZIVIR has been discontinued for reasons other than symptoms of a hypersensitivity reaction, and if reinitiation of abacavir is under consideration, carefully evaluate the reason for discontinuation to ensure that the patient did not have symptoms of a hypersensitivity reaction. If the patient is of unknown HLA-B*5701 status, screening for the allele is recommended prior to reinitiation of TRIZIVIR.
If hypersensitivity cannot be ruled out, DO NOT reintroduce TRIZIVIR or any other abacavir-containing product. Even in the absence of the HLA-B*5701 allele, it is important to permanently discontinue abacavir and not rechallenge with abacavir if a hypersensitivity reaction cannot be ruled out on clinical grounds, due to the potential for a severe or even fatal reaction.
If symptoms consistent with hypersensitivity are not identified, reintroduction can be undertaken with continued monitoring for symptoms of a hypersensitivity reaction. Make patients aware that a hypersensitivity reaction can occur with reintroduction of abacavir and that abacavir reintroduction needs to be undertaken only if medical care can be readily accessed by the patient or others.
Risk Factor:HLA-B*5701 Allele: Trials have shown that carriage of the HLA-B*5701 allele is associated with a significantly increased risk of a hypersensitivity reaction to abacavir.
CNA106030 (PREDICT-1), a randomized, double-blind trial, evaluated the clinical utility of prospective HLA-B*5701 screening on the incidence of abacavir hypersensitivity reaction in abacavir-naive HIV-1-infected adults (n = 1,650). In this trial, use of pre-therapy screening for the HLA-B*5701 allele and exclusion of subjects with this allele reduced the incidence of clinically suspected abacavir hypersensitivity reactions from 7.8% (66/847) to 3.4% (27/803). Based on this trial, it is estimated that 61% of patients with the HLA-B*5701 allele will develop a clinically suspected hypersensitivity reaction during the course of abacavir treatment compared with 4% of patients who do not have the HLA-B*5701 allele.
Screening for carriage of the HLA-B*5701 allele is recommended prior to initiating treatment with abacavir. Screening is also recommended prior to reinitiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir. For HLA-B*5701-positive patients, initiating or reinitiating treatment with an abacavir-containing regimen is not recommended and should be considered only with close medical supervision and under exceptional circumstances where potential benefit outweighs the risk.
Skin patch testing is used as a research tool and should not be used to aid in the clinical diagnosis of abacavir hypersensitivity.
In any patient treated with abacavir, the clinical diagnosis of hypersensitivity reaction must remain the basis of clinical decision-making. Even in the absence of the HLA-B*5701 allele, it is important to permanently discontinue abacavir and not rechallenge with abacavir if a hypersensitivity reaction cannot be ruled out on clinical grounds, due to the potential for a severe or even fatal reaction.
5.2 Hematologic Toxicity/Bone Marrow Suppression
Zidovudine, a component of TRIZIVIR, has been associated with hematologic toxicity including neutropenia and anemia, particularly in patients with advanced HIV-1 disease. TRIZIVIR should be used with caution in patients who have bone marrow compromise evidenced by granulocyte count less than 1,000 cells/mm3 or hemoglobin less than 9.5 g/dL.
Frequent blood counts are strongly recommended in patients with advanced HIV-1 disease who are treated with TRIZIVIR. Periodic blood counts are recommended for other HIV-1-infected patients. If anemia or neutropenia develops, dosage interruption may be needed.
5.3 Myopathy
Myopathy and myositis, with pathological changes similar to that produced by HIV-1 disease, have been associated with prolonged use of zidovudine, and therefore may occur with therapy with TRIZIVIR.
5.4 Lactic Acidosis/Hepatomegaly With Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including abacavir, lamivudine, zidovudine, and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering TRIZIVIR to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with TRIZIVIR should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.5 Patients With HIV-1 and Hepatitis B Virus Co-infection
Posttreatment Exacerbations of Hepatitis: In clinical trials in non-HIV-1-infected subjects treated with lamivudine for chronic HBV, clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of lamivudine. These exacerbations have been detected primarily by serum ALT elevations in addition to re-emergence of hepatitis B viral DNA (HBV DNA). Although most events appear to have been self-limited, fatalities have been reported in some cases. Similar events have been reported from post-marketing experience after changes from lamivudine-containing HIV-1 treatment regimens to non-lamivudine-containing regimens in patients infected with both HIV-1 and HBV. The causal relationship to discontinuation of lamivudine treatment is unknown. Patients should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. There is insufficient evidence to determine whether reinitiation of lamivudine alters the course of posttreatment exacerbations of hepatitis.
Emergence of Lamivudine-Resistant HBV: Safety and efficacy of lamivudine have not been established for treatment of chronic hepatitis B in subjects dually infected with HIV-1 and HBV. In non-HIV-infected subjects treated with lamivudine for chronic hepatitis B, emergence of lamivudine-resistant HBV has been detected and has been associated with diminished treatment response (see full prescribing information for EPIVIR-HBV® [lamivudine] for additional information). Emergence of hepatitis B virus variants associated with resistance to lamivudine has also been reported in HIV-1-infected subjects who have received lamivudine-containing antiretroviral regimens in the presence of concurrent infection with hepatitis B virus.
5.6 Use With Interferon- and Ribavirin-Based Regimens
In vitro studies have shown ribavirin can reduce the phosphorylation of pyrimidine nucleoside analogues such as lamivudine and zidovudine. Although no evidence of a pharmacokinetic or pharmacodynamic interaction (e.g., loss of HIV-1/HCV virologic suppression) was seen when ribavirin was coadministered with lamivudine or zidovudine in HIV-1/HCV co-infected subjects [see Clinical Pharmacology (12.3)], hepatic decompensation (some fatal) has occurred in HIV-1/HCV co-infected subjects receiving combination antiretroviral therapy for HIV-1 and interferon alfa with or without ribavirin. Patients receiving interferon alfa with or without ribavirin and TRIZIVIR should be closely monitored for treatment-associated toxicities, especially hepatic decompensation, neutropenia, and anemia. Discontinuation of TRIZIVIR should be considered as medically appropriate. Dose reduction or discontinuation of interferon alfa, ribavirin, or both should also be considered if worsening clinical toxicities are observed, including hepatic decompensation (e.g., Child-Pugh greater than 6) (see the complete prescribing information for interferon and ribavirin).
Exacerbation of anemia has been reported in HIV-1/HCV co-infected patients receiving ribavirin and zidovudine. Coadministration of ribavirin and TRIZIVIR is not advised.
5.7 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including TRIZIVIR. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.8 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.9 Myocardial Infarction
In a published prospective, observational, epidemiological trial designed to investigate the rate of myocardial infarction in patients on combination antiretroviral therapy, the use of abacavir within the previous 6 months was correlated with an increased risk of myocardial infarction (MI).1 In a sponsor-conducted pooled analysis of clinical trials, no excess risk of myocardial infarction was observed in abacavir-treated subjects as compared with control subjects. In totality, the available data from the observational cohort and from clinical trials are inconclusive.
As a precaution, the underlying risk of coronary heart disease should be considered when prescribing antiretroviral therapies, including abacavir, and action taken to minimize all modifiable risk factors (e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking).
5.10 Therapy Experienced Patients
In clinical trials, subjects with prolonged prior nucleoside reverse transcriptase inhibitor (NRTI) exposure or who had HIV-1 isolates that contained multiple mutations conferring resistance to NRTIs had limited response to abacavir. The potential for cross-resistance between abacavir and other NRTIs should be considered when choosing new therapeutic regimens in therapy-experienced patients [see Clinical Pharmacology (12.4)].
5.11 Use With Other Abacavir-, Lamivudine-, Zidovudine-, and/or Emtricitabine-Containing Products
TRIZIVIR is a fixed-dose combination of abacavir, lamivudine, and zidovudine and is intended only for patients whose regimen would otherwise include these 3 components. TRIZIVIR should not be administered concomitantly with other abacavir-, lamivudine-, or zidovudine-containing products including ZIAGEN (abacavir sulfate) Tablets and Oral Solution, EPIVIR® (lamivudine) Tablets and Oral Solution, EPIVIR-HBV (lamivudine) Tablets and Oral Solution, RETROVIR® (zidovudine) Tablets, Capsules, Syrup, and IV Infusion, COMBIVIR® (lamivudine and zidovudine) Tablets, EPZICOM® (abacavir sulfate and lamivudine) Tablets; or emtricitabine‑containing products, including ATRIPLA® (efavirenz/emtricitabine/tenofovir disoproxil fumarate) Tablets, EMTRIVA® (emtricitabine) Capsules and Oral Solution, TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) Tablets, or COMPLERA® (emtricitabine/rilpirivine/tenofovir disoproxil fumarate) Tablets.
The complete prescribing information for all agents being considered for use with TRIZIVIR should be consulted before combination therapy with TRIZIVIR is initiated.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Serious and sometimes fatal hypersensitivity reactions [see Boxed Warning, Warnings and Precautions (5.1)].
- •
- Hematologic toxicity, including neutropenia and anemia [see Boxed Warning, Warnings and Precautions (5.2)].
- •
- Symptomatic myopathy [see Boxed Warning, Warnings and Precautions (5.3)].
- •
- Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning, Warnings and Precautions (5.4)].
- •
- Acute exacerbations of hepatitis B [see Boxed Warning, Warnings and Precautions (5.5)].
- •
- Hepatic decompensation in patients co-infected with HIV-1 and hepatitis C [see Warnings and Precautions (5.6)].
- •
- Exacerbation of anemia in HIV-1/HCV co-infected patients receiving ribavirin and zidovudine [see Warnings and Precautions (5.6)].
- •
- Immune reconstitution syndrome [see Warnings and Precautions (5.7)].
- •
- Fat redistribution [see Warnings and Precautions (5.8)].
- •
- Myocardial infarction [see Warnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Treatment-emergent clinical adverse reactions (rated by the investigator as moderate or severe) with a frequency greater than or equal to 5% during therapy with abacavir 300 mg twice daily, lamivudine 150 mg twice daily, and zidovudine 300 mg twice daily compared with indinavir 800 mg 3 times daily, lamivudine 150 mg twice daily, and zidovudine 300 mg twice daily from CNA3005 are listed in Table 1.
Table 1. Treatment-Emergent (All Causality) Adverse Reactions of at Least Moderate Intensity (Grades 2-4, ≥5% Frequency) in Therapy-Naive Adults (CNA3005) Through 48 Weeks of Treatment Adverse Reaction
ZIAGEN plus
Lamivudine/Zidovudine
(n = 262)
Indinavir plus
Lamivudine/Zidovudine
(n = 264)
Nausea
19%
17%
Headache
13%
9%
Malaise and fatigue
12%
12%
Nausea and vomiting
10%
10%
Hypersensitivity reaction
8%
2%
Diarrhea
7%
5%
Fever and/or chills
6%
3%
Depressive disorders
6%
4%
Musculoskeletal pain
5%
7%
Skin rashes
5%
4%
Ear/nose/throat infections
5%
4%
Viral respiratory infections
5%
5%
Anxiety
5%
3%
Renal signs/symptoms
<1%
5%
Pain (non-site-specific)
<1%
5%
Five subjects receiving abacavir in CNA3005 experienced worsening of pre-existing depression compared to none in the indinavir arm. The background rates of pre-existing depression were similar in the 2 treatment arms.
Laboratory Abnormalities: Laboratory abnormalities in CNA3005 are listed in Table 2.
Table 2. Treatment-Emergent Laboratory Abnormalities (Grades 3/4) in CNA3005 Grade 3/4 Laboratory Abnormalities
Number of Subjects by Treatment Group
ZIAGEN plus
Lamivudine/Zidovudine
(n = 262)
Indinavir plus
Lamivudine/Zidovudine
(n = 264)
Elevated CPK (>4 x ULN)
18 (7%)
18 (7%)
ALT (>5.0 x ULN)
16 (6%)
16 (6%)
Neutropenia (<750/mm3)
13 (5%)
13 (5%)
Hypertriglyceridemia (>750 mg/dL)
5 (2%)
3 (1%)
Hyperamylasemia (>2.0 x ULN)
5 (2%)
1 (<1%)
Hyperglycemia (>13.9 mmol/L)
2 (<1%)
2 (<1%)
Anemia (Hgb ≤6.9 g/dL)
0 (0%)
3 (1%)
ULN = Upper limit of normal.
n = Number of patients assessed.
Other Adverse Events: In addition to adverse reactions in Tables 1 and 2, other adverse events observed in the expanded access program for abacavir were pancreatitis and increased GGT.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following reactions have been identified during postmarketing use of abacavir, lamivudine, and/or zidovudine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to abacavir, lamivudine and/or zidovudine.
Abacavir:
Cardiovascular: Myocardial infarction.
Skin: Suspected Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in patients receiving abacavir primarily in combination with medications known to be associated with SJS and TEN, respectively. Because of the overlap of clinical signs and symptoms between hypersensitivity to abacavir and SJS and TEN, and the possibility of multiple drug sensitivities in some patients, abacavir should be discontinued and not restarted in such cases.
There have also been reports of erythema multiforme with abacavir use.
Abacavir, Lamivudine, and/or Zidovudine:
Body as a Whole: Redistribution/accumulation of body fat [see Warnings and Precautions (5.8)].
Cardiovascular: Cardiomyopathy.
Digestive: Stomatitis.
Endocrine and Metabolic: Gynecomastia, hyperglycemia.
Gastrointestinal: Anorexia and/or decreased appetite, abdominal pain, dyspepsia, oral mucosal pigmentation.
General: Vasculitis, weakness.
Hemic and Lymphatic: Aplastic anemia, anemia (including pure red cell aplasia and severe anemias progressing on therapy), lymphadenopathy, splenomegaly, thrombocytopenia.
Hepatic: Lactic acidosis and hepatic steatosis [see Warnings and Precautions (5.4)], elevated bilirubin, elevated transaminases, posttreatment exacerbation of hepatitis B [see Warnings and Precautions (5.5)].
Hypersensitivity: Sensitization reactions (including anaphylaxis), urticaria.
Musculoskeletal: Arthralgia, myalgia, muscle weakness, CPK elevation, rhabdomyolysis.
Nervous: Dizziness, paresthesia, peripheral neuropathy, seizures.
Psychiatric: Insomnia and other sleep disorders.
Respiratory: Abnormal breath sounds/wheezing.
Skin: Alopecia, erythema multiforme, Stevens-Johnson syndrome.
-
7 DRUG INTERACTIONS
- •
- No drug interaction trials have been conducted using TRIZIVIR Tablets [see Clinical Pharmacology (12.3)].
7.1 Antiretroviral Agents
Zidovudine:Stavudine: Concomitant use of zidovudine with stavudine should be avoided since an antagonistic relationship has been demonstrated in vitro.
Nucleoside Analogues Affecting DNA Replication: Some nucleoside analogues affecting DNA replication, such as ribavirin, antagonize the in vitro antiviral activity of zidovudine against HIV-1; concomitant use of such drugs should be avoided.
7.2 Doxorubicin
Zidovudine: Concomitant use of zidovudine with doxorubicin should be avoided since an antagonistic relationship has been demonstrated in vitro.
7.3 Ethanol
Abacavir: Abacavir has no effect on the pharmacokinetic properties of ethanol. Ethanol decreases the elimination of abacavir causing an increase in overall exposure [see Clinical Pharmacology (12.3)].
7.4 Hematologic/Bone Marrow Suppressive/Cytotoxic Agents
Zidovudine: Coadministration of ganciclovir, interferon alfa, ribavirin, and other bone marrow suppressive or cytotoxic agents may increase the hematologic toxicity of zidovudine.
7.5 Interferon- and Ribavirin-Based Regimens
Lamivudine: Although no evidence of a pharmacokinetic or pharmacodynamic interaction (e.g., loss of HIV-1/HCV virologic suppression) was seen when ribavirin was coadministered with lamivudine in HIV-1/HCV co-infected subjects, hepatic decompensation (some fatal) has occurred in HIV-1/HCV co-infected subjects receiving combination antiretroviral therapy for HIV-1 and interferon alfa with or without ribavirin [see Warnings and Precautions (5.6), Clinical Pharmacology (12.3)].
7.6 Methadone
Abacavir: The addition of methadone has no clinically significant effect on the pharmacokinetic properties of abacavir. In a trial of 11 HIV-1-infected subjects receiving methadone-maintenance therapy with 600 mg of ZIAGEN twice daily (twice the currently recommended dose), oral methadone clearance increased [see Clinical Pharmacology (12.3)]. This alteration will not result in a methadone dose modification in the majority of patients; however, an increased methadone dose may be required in a small number of patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
TRIZIVIR: Pregnancy Category C. There are no adequate and well-controlled studies of TRIZIVIR in pregnant women. Reproduction studies with abacavir, lamivudine, and zidovudine have been performed in animals (see Abacavir, Lamivudine, and Zidovudine sections below). TRIZIVIR should be used during pregnancy only if the potential benefits outweigh the risks.
Abacavir: Studies in pregnant rats showed that abacavir is transferred to the fetus through the placenta. Fetal malformations (increased incidences of fetal anasarca and skeletal malformations) and developmental toxicity (depressed fetal body weight and reduced crown-rump length) were observed in rats at a dose which produced 35 times the human exposure, based on AUC. Embryonic and fetal toxicities (increased resorptions, decreased fetal body weights) and toxicities to the offspring (increased incidence of stillbirth and lower body weights) occurred at half of the above-mentioned dose in separate fertility studies conducted in rats. In the rabbit, no developmental toxicity and no increases in fetal malformations occurred at doses that produced 8.5 times the human exposure at the recommended dose based on AUC.
Lamivudine: Studies in pregnant rats showed that lamivudine is transferred to the fetus through the placenta. Reproduction studies with orally administered lamivudine have been performed in rats and rabbits at doses producing plasma levels up to approximately 35 times that for the recommended adult HIV dose. No evidence of teratogenicity due to lamivudine was observed. Evidence of early embryolethality was seen in the rabbit at exposure levels similar to those observed in humans, but there was no indication of this effect in the rat at exposure levels up to 35 times those in humans.
Zidovudine: Reproduction studies with orally administered zidovudine in the rat and in the rabbit at doses up to 500 mg/kg/day revealed no evidence of teratogenicity with zidovudine. Zidovudine treatment resulted in embryo/fetal toxicity as evidenced by an increase in the incidence of fetal resorptions in rats given 150 or 450 mg/kg/day and rabbits given 500 mg/kg/day. The doses used in the teratology studies resulted in peak zidovudine plasma concentrations (after one half of the daily dose) in rats 66 to 226 times, and in rabbits 12 to 87 times, mean steady-state peak human plasma concentrations (after one sixth of the daily dose) achieved with the recommended daily dose (100 mg every 4 hours). In an additional teratology study in rats, a dose of 3,000 mg/kg/day (very near the oral median lethal dose in rats of approximately 3,700 mg/kg) caused marked maternal toxicity and an increase in the incidence of fetal malformations. This dose resulted in peak zidovudine plasma concentrations 350 times peak human plasma concentrations. No evidence of teratogenicity was seen in this experiment at doses of 600 mg/kg/day or less. Two rodent carcinogenicity studies were conducted [see Nonclinical Toxicology (13.1)].
Antiretroviral Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to TRIZIVIR or other antiretroviral agents, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
8.3 Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV infection.
Abacavir, Lamivudine, and Zidovudine: Lamivudine and zidovudine are excreted in human breast milk; abacavir and lamivudine are secreted into the milk of lactating rats.
Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving TRIZIVIR.
8.4 Pediatric Use
TRIZIVIR is not intended for use in pediatric patients and is not recommended in adolescents who weigh less than 40 kg because it is a fixed-dose tablet that cannot be adjusted for these patient populations.
Therapy-Experienced Pediatric Trial: A randomized, double-blind trial, CNA3006, compared ZIAGEN plus lamivudine and zidovudine versus lamivudine and zidovudine in pediatric subjects, most of whom were extensively pretreated with nucleoside analogue antiretroviral agents. Subjects in this trial had a limited response to abacavir.
8.5 Geriatric Use
Clinical studies of abacavir, lamivudine, and zidovudine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Dosage and Administration (2.3), Use in Specific Populations (8.6)].
-
10 OVERDOSAGE
Abacavir: There is no known antidote for abacavir. It is not known whether abacavir can be removed by peritoneal dialysis or hemodialysis.
Lamivudine: One case of an adult ingesting 6 grams of lamivudine was reported; there were no clinical signs or symptoms noted and hematologic tests remained normal. It is not known whether lamivudine can be removed by peritoneal dialysis or hemodialysis.
Zidovudine: Acute overdoses of zidovudine have been reported in pediatric patients and adults. These involved exposures up to 50 grams. The only consistent findings were nausea and vomiting. Other reported occurrences included headache, dizziness, drowsiness, lethargy, and confusion. Hematologic changes were transient. All patients recovered. Hemodialysis and peritoneal dialysis appear to have a negligible effect on the removal of zidovudine, while elimination of its primary metabolite, 3′-azido-3′-deoxy-5′-O-β-D-glucopyranuronosylthymidine (GZDV), is enhanced.
-
11 DESCRIPTION
TRIZIVIR: TRIZIVIR Tablets contain the following 3 synthetic nucleoside analogues: abacavir sulfate (ZIAGEN), lamivudine (also known as EPIVIR or 3TC), and zidovudine (also known as RETROVIR, azidothymidine, or ZDV) with inhibitory activity against HIV-1.
TRIZIVIR Tablets are for oral administration. Each film-coated tablet contains the active ingredients 300 mg of abacavir as abacavir sulfate, 150 mg of lamivudine, and 300 mg of zidovudine, and the inactive ingredients magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The tablets are coated with a film (OPADRY® green 03B11434) that is made of FD&C Blue No. 2, hypromellose, polyethylene glycol, titanium dioxide, and yellow iron oxide.
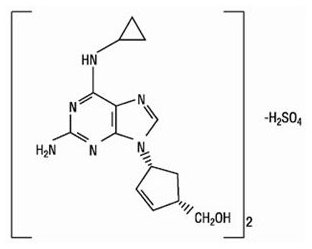
Abacavir Sulfate: The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). Abacavir sulfate is the enantiomer with 1S, 4R absolute configuration on the cyclopentene ring. It has a molecular formula of (C14H18N6O)2•H2SO4 and a molecular weight of 670.76 daltons. It has the following structural formula:
Abacavir sulfate is a white to off-white solid with a solubility of approximately 77 mg/mL in distilled water at 25°C.
In vivo, abacavir sulfate dissociates to its free base, abacavir. In this insert, all dosages for ZIAGEN (abacavir sulfate) are expressed in terms of abacavir.
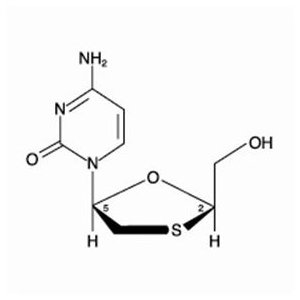
Lamivudine: The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2′,3′-dideoxy, 3′-thiacytidine. It has a molecular formula of C8H11N3O3S and a molecular weight of 229.3 daltons. It has the following structural formula:
Lamivudine is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20°C.
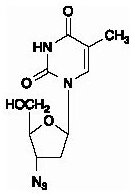
Zidovudine: The chemical name of zidovudine is 3′-azido-3′-deoxythymidine. It has a molecular formula of C10H13N5O4 and a molecular weight of 267.24 daltons. It has the following structural formula:
Zidovudine is a white to beige, crystalline solid with a solubility of 20.1 mg/mL in water at 25°C.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Pharmacokinetics in Adults:TRIZIVIR: In a single-dose, 3-way crossover bioavailability trial of 1 TRIZIVIR Tablet versus 1 ZIAGEN Tablet (300 mg), 1 EPIVIR Tablet (150 mg), plus 1 RETROVIR Tablet (300 mg) administered simultaneously in healthy subjects (n = 24), there was no difference in the extent of absorption, as measured by the area under the plasma concentration-time curve (AUC) and maximal peak concentration (Cmax), of all 3 components. One TRIZIVIR Tablet was bioequivalent to 1 ZIAGEN Tablet (300 mg), 1 EPIVIR Tablet (150 mg), plus 1 RETROVIR Tablet (300 mg) following single-dose administration to fasting healthy subjects (n = 24).
Abacavir: Following oral administration, abacavir is rapidly absorbed and extensively distributed. Binding of abacavir to human plasma proteins is approximately 50%. Binding of abacavir to plasma proteins was independent of concentration. Total blood and plasma drug-related radioactivity concentrations are identical, demonstrating that abacavir readily distributes into erythrocytes. The primary routes of elimination of abacavir are metabolism by alcohol dehydrogenase to form the 5′-carboxylic acid and glucuronyl transferase to form the 5′-glucuronide.
Lamivudine: Following oral administration, lamivudine is rapidly absorbed and extensively distributed. Binding to plasma protein is low. Approximately 70% of an intravenous dose of lamivudine is recovered as unchanged drug in the urine. Metabolism of lamivudine is a minor route of elimination. In humans, the only known metabolite is the trans-sulfoxide metabolite (approximately 5% of an oral dose after 12 hours).
Zidovudine: Following oral administration, zidovudine is rapidly absorbed and extensively distributed. Binding to plasma protein is low. Zidovudine is eliminated primarily by hepatic metabolism. The major metabolite of zidovudine is GZDV. GZDV AUC is about 3-fold greater than the zidovudine AUC. Urinary recovery of zidovudine and GZDV accounts for 14% and 74% of the dose following oral administration, respectively. A second metabolite, 3′-amino-3′-deoxythymidine (AMT), has been identified in plasma. The AMT AUC was one-fifth of the zidovudine AUC.
In humans, abacavir, lamivudine, and zidovudine are not significantly metabolized by cytochrome P450 enzymes.
The pharmacokinetic properties of abacavir, lamivudine, and zidovudine in fasting subjects are summarized in Table 3.
Table 3. Pharmacokinetic Parametersa for Abacavir, Lamivudine, and Zidovudine in Adults Parameter
Abacavir
Lamivudine
Zidovudine
Oral bioavailability (%)
86 ± 25
n = 6
86 ± 16
n = 12
64 ± 10
n = 5
Apparent volume of distribution (L/kg)
0.86 ± 0.15
n = 6
1.3 ± 0.4
n = 20
1.6 ± 0.6
n = 8
Systemic clearance (L/h/kg)
0.80 ± 0.24
n = 6
0.33 ± 0.06
n = 20
1.6 ± 0.6
n = 6
Renal clearance (L/h/kg)
.007 ± .008
n = 6
0.22 ± 0.06
n = 20
0.34 ± 0.05
n = 9
Elimination half-life (h)
1.45 ± 0.32
n = 20
5 to 7b
0.5 to 3b
a Data presented as mean ± standard deviation except where noted.
b Approximate range.
Effect of Food on Absorption of TRIZIVIR: Administration with food in a single-dose bioavailability trial resulted in lower Cmax, similar to results observed previously for the reference formulations. The average [90% CI] decrease in abacavir, lamivudine, and zidovudine Cmax was 32% [24% to 38%], 18% [10% to 25%], and 28% [13% to 40%], respectively, when administered with a high-fat meal, compared with administration under fasted conditions. Administration of TRIZIVIR with food did not alter the extent of abacavir, lamivudine, and zidovudine absorption (AUC), as compared with administration under fasted conditions (n = 24) [see Dosage and Administration (2.1)].
Special Populations: Renal Impairment: TRIZIVIR: Because lamivudine and zidovudine require dose adjustment in the presence of renal insufficiency, TRIZIVIR is not recommended for use in patients with creatinine clearance <50 mL/min [see Use in Specific Populations (8.6)].
Hepatic Impairment: TRIZIVIR: TRIZIVIR is contraindicated for patients with impaired hepatic function because TRIZIVIR is a fixed-dose combination and the dosage of the individual components cannot be adjusted. Abacavir is contraindicated in patients with moderate to severe hepatic impairment and dose reduction is required in patients with mild hepatic impairment.
Pregnancy: See Use in Specific Populations (8.1).
Abacavir and Lamivudine: No data are available on the pharmacokinetics of abacavir or lamivudine during pregnancy.
Zidovudine: Zidovudine pharmacokinetics have been studied in a Phase 1 trial of 8 women during the last trimester of pregnancy. As pregnancy progressed, there was no evidence of drug accumulation. The pharmacokinetics of zidovudine were similar to that of nonpregnant adults. Consistent with passive transmission of the drug across the placenta, zidovudine concentrations in neonatal plasma at birth were essentially equal to those in maternal plasma at delivery. Although data are limited, methadone maintenance therapy in 5 pregnant women did not appear to alter zidovudine pharmacokinetics. In a nonpregnant adult population, a potential for interaction has been identified [see Use in Specific Populations (8.1)].
Nursing Mothers: See Use in Specific Populations (8.3).
Abacavir: No data are available on the pharmacokinetics of abacavir in nursing mothers.
Lamivudine: Samples of breast milk obtained from 20 mothers receiving lamivudine monotherapy (300 mg twice daily) or combination therapy (150 mg lamivudine twice daily and 300 mg zidovudine twice daily) had measurable concentrations of lamivudine.
Zidovudine: After administration of a single dose of 200 mg zidovudine to 13 HIV‑1-infected women, the mean concentration of zidovudine was similar in human milk and serum [see Use in Specific Populations (8.3)].
Pediatric Patients: TRIZIVIR is not intended for use in pediatric patients. TRIZIVIR is not recommended in adolescents who weigh less than 40 kg because it is a fixed-dose tablet that cannot be dose adjusted for this patient population.
Geriatric Patients: The pharmacokinetics of abacavir, lamivudine, and zidovudine have not been studied in subjects over 65 years of age.
Gender:
Abacavir: A population pharmacokinetic analysis in HIV-1-infected male (n = 304) and female (n = 67) subjects showed no gender differences in abacavir AUC normalized for lean body weight.
Lamivudine and Zidovudine: A pharmacokinetic trial in healthy male (n = 12) and female (n = 12) subjects showed no gender differences in zidovudine exposure (AUC∞) or lamivudine (AUC∞) normalized for body weight.
Race:
Abacavir: There are no significant differences between blacks and Caucasians in abacavir pharmacokinetics.
Lamivudine: There are no significant racial differences in lamivudine pharmacokinetics.
Zidovudine: The pharmacokinetics of zidovudine with respect to race have not been determined.
Drug Interactions: The drug interactions described below are based on trials conducted with the individual nucleoside analogues.
Cytochrome P450: In humans, abacavir, lamivudine, and zidovudine are not significantly metabolized by cytochrome P450 enzymes; therefore, it is unlikely that clinically significant drug interactions will occur with drugs metabolized through these pathways.
Glucuronyl Transferase: Due to the common metabolic pathways of abacavir and zidovudine via glucuronyl transferase, 15 HIV-1-infected subjects were enrolled in a crossover trial evaluating single doses of abacavir (600 mg), lamivudine (150 mg), and zidovudine (300 mg) alone or in combination. Analysis showed no clinically relevant changes in the pharmacokinetics of abacavir with the addition of lamivudine or zidovudine or the combination of lamivudine and zidovudine. Lamivudine exposure (AUC decreased 15%) and zidovudine exposure (AUC increased 10%) did not show clinically relevant changes with concurrent abacavir.
Lamivudine and Zidovudine: No clinically significant alterations in lamivudine or zidovudine pharmacokinetics were observed in 12 asymptomatic HIV-1-infected adult subjects given a single dose of zidovudine (200 mg) in combination with multiple doses of lamivudine (300 mg q 12 h).
Methadone: In a trial of 11 HIV-1-infected subjects receiving methadone-maintenance therapy (40 mg and 90 mg daily), with 600 mg of ZIAGEN twice daily (twice the currently recommended dose), oral methadone clearance increased 22% (90% CI: 6% to 42%) [see Drug Interactions (7.6)].
Ribavirin: In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (e.g., plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (e.g., loss of HIV-1/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n = 18), stavudine (n = 10), or zidovudine (n = 6) were coadministered as part of a multi-drug regimen to HIV-1/HCV co-infected subjects [see Warnings and Precautions (5.6)].
The effects of other coadministered drugs on abacavir, lamivudine, or zidovudine are provided in Table 4.
Table 4. Effect of Coadministered Drugs on Abacavir, Lamivudine, and Zidovudine AUCa Note: ROUTINE DOSE MODIFICATION OF ABACAVIR, LAMIVUDINE, AND ZIDOVUDINE IS NOT WARRANTED WITH COADMINISTRATION OF THE FOLLOWING DRUGS. Drugs That May Alter Lamivudine Blood Concentrations
Coadministered Drug and Dose
Lamivudine Dose
n
Lamivudine Concentrations
Concentration of Coadministered Drug
AUC
Variability
Nelfinavir
750 mg q 8 h
x 7 to 10 days
single 150 mg
11
↑10%
95% CI:
1% to 20%
↔
Trimethoprim 160 mg/
Sulfamethoxazole
800 mg daily
x 5 days
single 300 mg
14
↑43%
90% CI:
32% to 55%
↔
Drugs That May Alter Zidovudine Blood Concentrations
Coadministered Drug and Dose
Zidovudine Dose
n
Zidovudine Concentrations
Concentration of Coadministered Drug
AUC
Variability
Atovaquone
750 mg q 12 h
with food
200 mg q 8 h
14
↑31%
Range
23% to 78%b
↔
Clarithromycin
500 mg twice daily
100 mg q 4 h x 7 days
4
↓12%
Range ↓34% to ↑14%
Not Reported
Fluconazole
400 mg daily
200 mg q 8 h
12
↑74%
95% CI:
54% to 98%
Not Reported
Methadone
30 to 90 mg daily
200 mg q 4 h
9
↑43%
Range
16% to 64%b
↔
Nelfinavir
750 mg q 8 h x 7 to
10 days
single 200 mg
11
↓35%
Range
28% to 41%
↔
Probenecid
500 mg q 6 h x
2 days
2 mg/kg q 8 h x 3 days
3
↑106%
Range
100% to 170%b
Not Assessed
Rifampin
600 mg daily x 14 days
200 mg q 8 h x 14 days
8
↓47%
90% CI: 41% to 53%
Not Assessed
Ritonavir
300 mg q 6 h x
4 days
200 mg q 8 h x 4 days
9
↓25%
95% CI:
15% to 34%
↔
Valproic acid
250 mg or 500 mg
q 8 h x 4 days
100 mg q 8 h x 4 days
6
↑80%
Range
64% to 130%b
Not Assessed
Drugs That May Alter Abacavir Blood Concentrations
Coadministered Drug and Dose
Abacavir Dose
n
Abacavir Concentrations
Concentration of Coadministered Drug
AUC
Variability
Ethanol
0.7 g/kg
single 600 mg
24
↑41%
90% CI:
35% to 48%
↔
↑ = Increase; ↓ = Decrease; ↔ = no significant change; AUC = area under the concentration versus time curve; CI = confidence interval.
a See Drug Interactions (7) for additional information on drug interactions.
b Estimated range of percent difference.
12.4 Microbiology
Mechanism of Action:Abacavir: Abacavir is a carbocyclic synthetic nucleoside analogue. Abacavir is converted by cellular enzymes to the active metabolite, carbovir triphosphate (CBV-TP), an analogue of deoxyguanosine-5′-triphosphate (dGTP). CBV-TP inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA. The lack of a 3′-OH group in the incorporated nucleotide analogue prevents the formation of the 5′ to 3′ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated. CBV-TP is a weak inhibitor of cellular DNA polymerases α, β, and γ.
Lamivudine: Lamivudine is a synthetic nucleoside analogue. Intracellularly, lamivudine is phosphorylated to its active 5′-triphosphate metabolite, lamivudine triphosphate (3TC-TP). The principal mode of action of 3TC-TP is inhibition of RT via DNA chain termination after incorporation of the nucleotide analogue. 3TC-TP is a weak inhibitor of cellular DNA polymerases α, β, and γ.
Zidovudine: Zidovudine is a synthetic nucleoside analogue. Intracellularly, zidovudine is phosphorylated to its active 5′-triphosphate metabolite, zidovudine triphosphate (ZDV-TP). The principal mode of action of ZDV-TP is inhibition of RT via DNA chain termination after incorporation of the nucleotide analogue. ZDV-TP is a weak inhibitor of the cellular DNA polymerases α and γ and has been reported to be incorporated into the DNA of cells in culture.
Antiviral Activity:Abacavir: The antiviral activity of abacavir against HIV-1 was evaluated against a T-cell tropic laboratory strain HIV-1IIIB in lymphoblastic cell lines, a monocyte/macrophage tropic laboratory strain HIV-1BaL in primary monocytes/macrophages, and clinical isolates in peripheral blood mononuclear cells. The concentration of drug necessary to effect viral replication by 50 percent (EC50) ranged from 3.7 to 5.8 μM (1 μM = 0.28 mcg/mL) and 0.07 to 1.0 μM against HIV-1IIIB and HIV-1BaL, respectively, and was 0.26 ± 0.18 μM against 8 clinical isolates. The EC50 values of abacavir against different HIV-1 clades (A-G) ranged from 0.0015 to 1.05 μM, and against HIV-2 isolates, from 0.024 to 0.49 μM. Abacavir had synergistic activity in cell culture in combination with the NRTI zidovudine, the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine, and the protease inhibitor (PI) amprenavir; and additive activity in combination with the NRTIs didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zalcitabine. Ribavirin (50 μM) had no effect on the anti–HIV-1 activity of abacavir in cell culture.
Lamivudine: The antiviral activity of lamivudine against HIV-1 was assessed in a number of cell lines (including monocytes and fresh human peripheral blood lymphocytes) using standard susceptibility assays. EC50 values (50% effective concentrations) were in the range of 0.003 to 15 μM (1 μM = 0.23 mcg/mL). HIV-1 from therapy-naive subjects with no amino acid substitutions associated with resistance gave median EC50 values of 0.429 µM (range: 0.200 to 2.007 µM) from Virco (n = 92 baseline samples from COLA40263) and 2.35 µM (1.37 to 3.68 µM) from Monogram Biosciences (n = 135 baseline samples from ESS30009). The EC50 values of lamivudine against different HIV-1 clades (A-G) ranged from 0.001 to 0.120 µM, and against HIV-2 isolates from 0.003 to 0.120 μM in peripheral blood mononuclear cells. Ribavirin (50 μM) decreased the anti-HIV-1 activity of lamivudine by 3.5-fold in MT-4 cells.
Zidovudine: The antiviral activity of zidovudine against HIV-1 was assessed in a number of cell lines (including monocytes and fresh human peripheral blood lymphocytes). The EC50 and EC90 values for zidovudine were 0.01 to 0.49 µM (1 μM = 0.27 mcg/mL) and 0.1 to 9 μM, respectively. HIV-1 from therapy-naive subjects with no amino acid substitutions associated with resistance gave median EC50 values of 0.011 µM (range: 0.005 to 0.110 µM) from Virco (n = 92 baseline samples from COLA40263) and 0.0017 µM (0.006 to 0.0340 µM) from Monogram Biosciences (n = 135 baseline samples from ESS30009). The EC50 values of zidovudine against different HIV-1 clades (A-G) ranged from 0.00018 to 0.02 μM, and against HIV-2 isolates from 0.00049 to 0.004 μM. In cell culture drug combination studies, zidovudine demonstrates synergistic activity with the NRTIs abacavir, didanosine, lamivudine, and zalcitabine; the NNRTIs delavirdine and nevirapine; and the PIs indinavir, nelfinavir, ritonavir, and saquinavir; and additive activity with interferon alfa. Ribavirin has been found to inhibit the phosphorylation of zidovudine in cell culture.
Resistance: HIV-1 isolates with reduced sensitivity to abacavir, lamivudine, or zidovudine have been selected in cell culture and were also obtained from subjects treated with abacavir, lamivudine, and zidovudine, or the combination of lamivudine and zidovudine.
Abacavir: Genotypic analysis of isolates selected in cell culture and recovered from abacavir-treated subjects demonstrated that amino acid substitutions K65R, L74V, Y115F, and M184V/I in HIV-1 RT contributed to abacavir resistance. In a trial of subjects receiving abacavir once or twice daily in combination with lamivudine and efavirenz once daily, 39% (7/18) of the isolates from subjects who experienced virologic failure in the abacavir once-daily arm had a >2.5-fold decrease in abacavir susceptibility with a median-fold decrease of 1.3 (range: 0.5 to 11) compared with 29% (5/17) of the failure isolates in the twice-daily arm with a median-fold decrease of 0.92 (range: 0.7 to 13).
Lamivudine: Genotypic analysis of isolates selected in cell culture and recovered from lamivudine-treated subjects showed that the resistance was due to a specific amino acid substitution in the HIV-1 RT at codon 184 changing the methionine to either valine or isoleucine (M184V/I).
Zidovudine: Genotypic analyses of the isolates selected in cell culture and recovered from zidovudine-treated subjects showed mutations in the HIV-1 RT gene resulting in 6 amino acid substitutions (M41L, D67N, K70R, L210W, T215Y or F, and K219Q) that confer zidovudine resistance. In general, higher levels of resistance were associated with greater number of mutations. In some subjects harboring zidovudine-resistant virus at baseline, phenotypic sensitivity to zidovudine was restored by 12 weeks of treatment with lamivudine and zidovudine. Combination therapy with lamivudine plus zidovudine delayed the emergence of substitutions conferring resistance to zidovudine.
Cross-Resistance: Cross-resistance has been observed among NRTIs.
Abacavir: Isolates containing abacavir resistance-associated amino acid substitutions, namely, K65R, L74V, Y115F, and M184V, exhibited cross-resistance to didanosine, emtricitabine, lamivudine, tenofovir, and zalcitabine in cell culture and in subjects. The K65R substitution can confer resistance to abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zalcitabine; the L74V substitution can confer resistance to abacavir, didanosine, and zalcitabine; and the M184V substitution can confer resistance to abacavir, didanosine, emtricitabine, lamivudine, and zalcitabine. An increasing number of thymidine analogue mutations (TAMs: M41L, D67N, K70R, L210W, T215Y/F, K219E/R/H/Q/N) is associated with a progressive reduction in abacavir susceptibility.
Lamivudine: Cross-resistance to abacavir, didanosine, tenofovir, and zalcitabine has been observed in some subjects harboring lamivudine-resistant HIV-1 isolates. In some subjects treated with zidovudine plus didanosine or zalcitabine, isolates resistant to multiple drugs, including lamivudine, have emerged (see under Zidovudine below). Cross-resistance between lamivudine and zidovudine has not been reported.
Zidovudine: In a trial of 167 HIV-infected subjects, isolates (n = 2) with multi-drug resistance to didanosine, lamivudine, stavudine, zalcitabine, and zidovudine were recovered from subjects treated for ≥1 year with zidovudine plus didanosine or zidovudine plus zalcitabine. The pattern of resistance-associated amino acid substitutions with such combination therapies was different (A62V, V75I, F77L, F116Y, Q151M) from the pattern with zidovudine monotherapy, with the Q151M substitution being most commonly associated with multi-drug resistance. The substitution at codon 151 in combination with substitutions at 62, 75, 77, and 116 results in a virus with reduced susceptibility to didanosine, lamivudine, stavudine, zalcitabine, and zidovudine. TAMs are selected by zidovudine and confer cross-resistance to abacavir, didanosine, stavudine, tenofovir, and zalcitabine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity:
Abacavir: Abacavir was administered orally at 3 dosage levels to separate groups of mice and rats in 2-year carcinogenicity studies. Results showed an increase in the incidence of malignant and non-malignant tumors. Malignant tumors occurred in the preputial gland of males and the clitoral gland of females of both species, and in the liver of female rats. In addition, non-malignant tumors also occurred in the liver and thyroid gland of female rats. These observations were made at systemic exposures in the range of 6 to 32 times the human exposure at the recommended dose. It is not known how predictive the results of rodent carcinogenicity studies may be for humans.
Lamivudine: Long-term carcinogenicity studies with lamivudine in mice and rats showed no evidence of carcinogenic potential at exposures up to 10 times (mice) and 58 times (rats) those observed in humans at the recommended therapeutic dose for HIV-1 infection.
Zidovudine: Zidovudine was administered orally at 3 dosage levels to separate groups of mice and rats (60 females and 60 males in each group). Initial single daily doses were 30, 60, and 120 mg/kg/day in mice and 80, 220, and 600 mg/kg/day in rats. The doses in mice were reduced to 20, 30, and 40 mg/kg/day after day 90 because of treatment-related anemia, whereas in rats only the high dose was reduced to 450 mg/kg per day on day 91 and then to 300 mg/kg/day on day 279.
In mice, 7 late-appearing (after 19 months) vaginal neoplasms (5 nonmetastasizing squamous cell carcinomas, 1 squamous cell papilloma, and 1 squamous polyp) occurred in animals given the highest dose. One late-appearing squamous cell papilloma occurred in the vagina of a middle-dose animal. No vaginal tumors were found at the lowest dose.
In rats, 2 late-appearing (after 20 months), nonmetastasizing vaginal squamous cell carcinomas occurred in animals given the highest dose. No vaginal tumors occurred at the low or middle dose in rats. No other drug-related tumors were observed in either sex of either species.
At doses that produced tumors in mice and rats, the estimated drug exposure (as measured by AUC) was approximately 3 times (mouse) and 24 times (rat) the estimated human exposure at the recommended therapeutic dose of 100 mg every 4 hours.
Two transplacental carcinogenicity studies were conducted in mice. One study administered zidovudine at doses of 20 mg/kg/day or 40 mg/kg/day from gestation day 10 through parturition and lactation with dosing continuing in offspring for 24 months postnatally. At these doses, exposures were approximately 3 times the estimated human exposure at the recommended doses. After 24 months at the 40-mg/kg/day dose, an increase in incidence of vaginal tumors was noted with no increase in tumors in the liver or lung or any other organ in either gender. These findings are consistent with results of the standard oral carcinogenicity study in mice, as described earlier. A second study administered zidovudine at maximum tolerated doses of 12.5 mg/day or 25 mg/day (~1,000 mg/kg nonpregnant body weight or ~450 mg/kg of term body weight) to pregnant mice from days 12 through 18 of gestation. There was an increase in the number of tumors in the lung, liver, and female reproductive tracts in the offspring of mice receiving the higher dose level of zidovudine.
It is not known how predictive the results of rodent carcinogenicity studies may be for humans.
Mutagenicity:
Abacavir: Abacavir induced chromosomal aberrations both in the presence and absence of metabolic activation in an in vitro cytogenetic study in human lymphocytes. Abacavir was mutagenic in the absence of metabolic activation, although it was not mutagenic in the presence of metabolic activation in an L5178Y/TK+/- mouse lymphoma assay. Abacavir was clastogenic in males and not clastogenic in females in an in vivo mouse bone marrow micronucleus assay. Abacavir was not mutagenic in bacterial mutagenicity assays in the presence and absence of metabolic activation.
Lamivudine: Lamivudine was mutagenic in an L5178Y/TK+/- mouse lymphoma assay and clastogenic in a cytogenetic assay using cultured human lymphocytes. Lamivudine was negative in a microbial mutagenicity assay, in an in vitro cell transformation assay, in a rat micronucleus test, in a rat bone marrow cytogenetic assay, and in an assay for unscheduled DNA synthesis in rat liver.
Zidovudine: Zidovudine was mutagenic in an L5178Y/TK+/- mouse lymphoma assay, positive in an in vitro cell transformation assay, clastogenic in a cytogenetic assay using cultured human lymphocytes, and positive in mouse and rat micronucleus tests after repeated doses. It was negative in a cytogenetic study in rats given a single dose.
Impairment of Fertility:
Abacavir: Abacavir had no adverse effects on the mating performance or fertility of male and female rats at a dose approximately 8 times the human exposure at the recommended dose based on body surface area comparisons.
Lamivudine: In a study of reproductive performance, lamivudine, administered to male and female rats at doses up to 130 times the usual adult dose based on body surface area considerations, revealed no evidence of impaired fertility judged by conception rates and no effect on the survival, growth, and development to weaning of the offspring.
Zidovudine: Zidovudine, administered to male and female rats at doses up to 7 times the usual adult dose based on body surface area considerations, had no effect on fertility judged by conception rates.
13.2 Animal Toxicology and/or Pharmacology
Myocardial degeneration was found in mice and rats following administration of abacavir for 2 years. The systemic exposures were equivalent to 7 to 24 times the expected systemic exposure in humans. The clinical relevance of this finding has not been determined.
-
14 CLINICAL STUDIES
The following trial was conducted with the individual components of TRIZIVIR [see Clinical Pharmacology (12.3)].
CNA3005 was a multicenter, double-blind, controlled trial in which 562 HIV-1-infected, therapy-naive adults were randomized to receive either ZIAGEN (300 mg twice daily) plus COMBIVIR (lamivudine 150 mg/zidovudine 300 mg twice daily), or indinavir (800 mg 3 times a day) plus COMBIVIR twice daily. The trial was stratified at randomization by pre-entry plasma HIV-1 RNA 10,000 to 100,000 copies/mL and plasma HIV-1 RNA >100,000 copies/mL. Trial participants were male (87%), Caucasian (73%), black (15%), and Hispanic (9%). At baseline the median age was 36 years, the median pretreatment CD4+ cell count was 360 cells/mm3, and median plasma HIV-1 RNA was 4.8 log10 copies/mL. Proportions of subjects with plasma HIV-1 RNA <400 copies/mL (using Roche AMPLICOR HIV-1 MONITOR® Test) through 48 weeks of treatment are summarized in Table 5.
Table 5. Outcomes of Randomized Treatment Through Week 48 (CNA3005) Outcome
ZIAGEN plus
Lamivudine/Zidovudine
(n = 262)
Indinavir plus
Lamivudine/Zidovudine
(n = 265)
Respondera
49%
50%
Virologic failureb
31%
28%
Discontinued due to adverse reactions
10%
12%
Discontinued due to other reasonsc
11%
10%
a Patients achieved and maintained confirmed HIV-1 RNA <400 copies/mL.
b Includes viral rebound and failure to achieve confirmed <400 copies/mL by Week 48.
c Includes consent withdrawn, lost to follow-up, protocol violations, those with missing data, clinical progression, and other.
Treatment response by plasma HIV-1 RNA strata is shown in Table 6.
Table 6. Proportions of Responders Through Week 48 By Screening Plasma HIV-1 RNA Levels (CNA3005) Screening
HIV-1 RNA
(copies/mL)
ZIAGEN plus
Lamivudine/Zidovudine
(n = 262)
Indinavir plus
Lamivudine/Zidovudine
(n = 265)
<400 copies/mL
n
<400 copies/mL
N
≥10,000 - ≤100,000
50%
166
48%
165
>100,000
48%
96
52%
100
In subjects with baseline viral load >100,000 copies/mL, percentages of subjects with HIV-1 RNA levels <50 copies/mL were 31% in the group receiving abacavir vs. 45% in the group receiving indinavir.
Through Week 48, an overall mean increase in CD4+ cell count of about 150 cells/mm3 was observed in both treatment arms. Through Week 48, 9 subjects (3.4%) in the group receiving abacavir sulfate (6 CDC classification C events and 3 deaths) and 3 subjects (1.5%) in the group receiving indinavir (2 CDC classification C events and 1 death) experienced clinical disease progression.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TRIZIVIR is available as tablets. Each tablet contains 300 mg of abacavir as abacavir sulfate, 150 mg of lamivudine, and 300 mg of zidovudine. The tablets are blue-green capsule-shaped, film-coated, and imprinted with GX LL1 on one side with no markings on the reverse side.
They are supplied by State of Florida DOH Central Pharmacy as follows:
NDC Strength Quantity/Form Color Source Prod. Code 53808-0990-1 300 mg / 150 mg / 300 mg 30 Tablets in a Blister Pack blue-green 49702-217 Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature).
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
Hypersensitivity Reaction: Inform patients:
- •
- that a Medication Guide and Warning Card summarizing the symptoms of the abacavir hypersensitivity reaction and other product information will be dispensed by the pharmacist with each new prescription and refill of TRIZIVIR, and encourage the patient to read the Medication Guide and Warning Card every time to obtain any new information that may be present about TRIZIVIR. (The complete text of the Medication Guide is reprinted at the end of this document.)
- •
- to carry the Warning Card with them.
- •
- how to identify a hypersensitivity reaction[see Warnings and Precautions (5.1), Medication Guide].
- •
- that if they develop symptoms consistent with a hypersensitivity reaction they should call their doctor right away to determine if they should stop taking TRIZIVIR.
- •
- that a hypersensitivity reaction can worsen and lead to hospitalization or death if TRIZIVIR is not immediately discontinued.
- •
- to not restart TRIZIVIR or any other abacavir-containing product following a hypersensitivity reaction because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
- •
- that a hypersensitivity reaction is usually reversible if it is detected promptly and TRIZIVIR is stopped right away.
- •
- that if they have interrupted TRIZIVIR for reasons other than symptoms of hypersensitivity (for example, those who have an interruption in drug supply), a serious or fatal hypersensitivity reaction may occur with reintroduction of abacavir.
- •
- to not restart TRIZIVIR or any other abacavir-containing product without medical consultation and that restarting abacavir needs to be undertaken only if medical care can be readily accessed by the patient or others.
- •
- TRIZIVIR should not be coadministered with ATRIPLA, COMBIVIR, COMPLERA, EMTRIVA, EPIVIR, EPIVIR-HBV, EPZICOM, RETROVIR (zidovudine), TRUVADA, or ZIAGEN.
Neutropenia and Anemia: Patients should be informed that the important toxicities associated with zidovudine are neutropenia and/or anemia. They should be told of the extreme importance of having their blood counts followed closely while on therapy, especially for patients with advanced HIV-1 disease [see Warnings and Precautions (5.2)].
Myopathy: Patients should be informed that myopathy and myositis with pathological changes, similar to that produced by HIV-1 disease, have been associated with prolonged use of zidovudine [see Warnings and Precautions (5.3)].
Lactic Acidosis/Hepatomegaly: Inform patients that some HIV medicines, including TRIZIVIR, can cause a rare, but serious condition called lactic acidosis with liver enlargement (hepatomegaly) [see Warnings and Precautions (5.4)].
HIV-1/ HBV Co-Infection: Patients co-infected with HIV-1 and HBV should be informed that deterioration of liver disease has occurred in some cases when treatment with lamivudine was discontinued. Patients should be advised to discuss any changes in regimen with their physician [see Warnings and Precautions (5.5)].
HIV-1/HCV Co-Infection: Patients with HIV-1/HCV co-infection should be informed that hepatic decompensation (some fatal) has occurred in HIV-1/HCV co-infected patients receiving combination antiretroviral therapy for HIV-1 and interferon alfa with or without ribavirin [see Warnings and Precautions (5.6)].
Redistribution/Accumulation of Body Fat: Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time [see Warnings and Precautions (5.8)].
Information About HIV-1 Infection: TRIZIVIR is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using TRIZIVIR.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- •
- Do not share needles or other injection equipment.
- •
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- •
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- •
- Do not breastfeed. Lamivudine and zidovudine are excreted in human breast milk. It is not known if abacavir can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Patients should be informed to take all HIV medications exactly as prescribed.
COMBIVIR, EPIVIR, EPZICOM, RETROVIR, TRIZIVIR, and ZIAGEN are registered trademarks of ViiV Healthcare.
Other brands are trademarks of their respective owners and are not trademarks of ViiV Healthcare. The makers of these brands are not affiliated with and do not endorse ViiV Healthcare or its products.
Manufactured for:
ViiV Healthcare
Research Triangle Park, NC 27709
by:
GlaxoSmithKline
Research Triangle Park, NC 27709
Lamivudine is manufactured under agreement from
Shire Pharmaceuticals Group plc
Basingstoke, UK
©2013, ViiV Healthcare. All rights reserved.
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
-
MEDICATION GUIDE
MEDICATION GUIDE
TRIZIVIR® (TRY-zih-veer)
(abacavir sulfate, lamivudine, and zidovudine
Tablets
Read this Medication Guide before you start taking TRIZIVIR and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Be sure to carry your TRIZIVIR Warning Card with you at all times.
What is the most important information I should know about TRIZIVIR?
1.Serious allergic reaction (hypersensitivity reaction). TRIZIVIR contains abacavir (also contained in ZIAGEN® and EPZICOM®). Patients taking TRIZIVIR may have a serious allergic reaction (hypersensitivity reaction) that can cause death. Your risk of this allergic reaction is much higher if you have a gene variation called HLA-B*5701. Your healthcare provider can determine with a blood test if you have this gene variation.
If you get a symptom from 2 or more of the following groups while taking TRIZIVIR, call your healthcare provider right away to find out if you should stop taking TRIZIVIR.
Symptom(s)
Group 1
Fever
Group 2
Rash
Group 3
Nausea, vomiting, diarrhea, abdominal (stomach area) pain
Group 4
Generally ill feeling, extreme tiredness, or achiness
Group 5
Shortness of breath, cough, sore throat
A list of these symptoms is on the Warning Card your pharmacist gives you. Carry this Warning Card with you at all times.
If you stop TRIZIVIR because of an allergic reaction, never take TRIZIVIR(abacavir sulfate, lamivudine, and zidovudine)or any other abacavir-containing medicine (ZIAGEN and EPZICOM) again. If you take TRIZIVIR or any other abacavir-containing medicine again after you have had an allergic reaction, within hours you may get life-threatening symptoms that may include very low blood pressure or death. If you stop TRIZIVIR, for any other reason, even for a few days, and you are not allergic to TRIZIVIR, talk with your healthcare provider before taking it again. Taking TRIZIVIR again can cause a serious allergic or life-threatening reaction, even if you never had an allergic reaction to it before.
If your healthcare provider tells you that you can take TRIZIVIR again, start taking it when you are around medical help or people who can call a healthcare provider if you need one.
2. Blood problems. RETROVIR®, one of the medicines in TRIZIVIR, can cause serious blood cell problems. These include reduced numbers of white blood cells (neutropenia) and extremely reduced numbers of red blood cells (anemia). These blood cell problems are especially likely to happen in patients with advanced human immunodeficiency virus (HIV) disease or AIDS. Your doctor should be checking your blood cell counts regularly while you are taking TRIZIVIR. This is especially important if you have advanced HIV or AIDS. This is to make sure that any blood cell problems are found quickly.
3. Lactic Acidosis (buildup of acid in the blood). Some human immunodeficiency virus (HIV) medicines, including TRIZIVIR, can cause a rare but serious condition called lactic acidosis. Lactic acidosis is a serious medical emergency that can cause death and must be treated in the hospital.
Call your healthcare provider right away if you get any of the following signs or symptoms of lactic acidosis:
- •
- you feel very weak or tired
- •
- you have unusual (not normal) muscle pain
- •
- you have trouble breathing
- •
- you have stomach pain with nausea and vomiting
- •
- you feel cold, especially in your arms and legs
- •
- you feel dizzy or light-headed
- •
- you have a fast or irregular heartbeat
4. Serious liver problems. Some people who have taken medicines like TRIZIVIR have developed serious liver problems called hepatotoxicity, with liver enlargement (hepatomegaly) and fat in the liver (steatosis). Hepatomegaly with steatosis is a serious medical emergency that can cause death.
Call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
- •
- your skin or the white part of your eyes turns yellow (jaundice)
- •
- your urine turns dark
- •
- your bowel movements (stools) turn light in color
- •
- you don’t feel like eating food for several days or longer
- •
- you feel sick to your stomach (nausea)
- •
- you have lower stomach area (abdominal) pain
You may be more likely to get lactic acidosis or serious liver problems if you are female, very overweight, or have been taking nucleoside analogue medicines for a long time.
5. Use with interferon and ribavirin-based regimens. Worsening of liver disease (sometimes resulting in death) has occurred in patients infected with both HIV and hepatitis C virus who are taking anti-HIV medicines and are also being treated for hepatitis C with interferon with or without ribavirin. If you are taking TRIZIVIR as well as interferon with or without ribavirin and you experience side effects, be sure to tell your healthcare provider.
6. If you have HIV and hepatitis B virus infection, your hepatitis B virus infection may get worse if you stop taking TRIZIVIR.
- •
- Take TRIZIVIR exactly as prescribed.
- •
- Do not run out of TRIZIVIR.
- •
- Do not stop TRIZIVIR without talking to your healthcare provider.
Your healthcare provider should monitor your health and do regular blood tests to check your liver if you stop taking TRIZIVIR.
7. Muscle weakness (myopathy). RETROVIR, one of the medicines in TRIZIVIR, can cause muscle weakness. This can be a serious problem.
What is TRIZIVIR?
TRIZIVIR is a prescription medicine used to treat HIV infection. TRIZIVIR contains 3 medicines: abacavir (ZIAGEN), lamivudine or 3TC (EPIVIR®), and zidovudine, AZT, or ZDV (RETROVIR). All 3 of these medicines are called nucleoside analogue reverse transcriptase inhibitors (NRTIs). When used together, they help lower the amount of HIV in your blood.
- •
- TRIZIVIR does not cure HIV infection or AIDS.
- •
- It is not known if TRIZIVIR will help you live longer or have fewer of the medical problems that people get with HIV or AIDS.
- •
- It is very important that you see your healthcare provider regularly while you are taking TRIZIVIR.
Who should not take TRIZIVIR?
Do not take TRIZIVIR if you:
- •
- are allergic to abacavir or any of the ingredients in TRIZIVIR. See the end of this Medication Guide for a complete list of ingredients in TRIZIVIR.
- •
- have certain liver problems.
- •
- are an adolescent who weighs less than 90 pounds.
What should I tell my healthcare provider before taking TRIZIVIR?
Before you take TRIZIVIR, tell your healthcare provider if you:
- •
- have been tested and know whether or not you have a particular gene variation called HLA-B*5701.
- •
- have hepatitis B virus infection or have other liver problems.
- •
- have kidney problems.
- •
- have low blood cell counts (bone marrow problem). Ask your doctor if you are not sure.
- •
- have heart problems, smoke, or have diseases that increase your risk of heart disease such as high blood pressure, high cholesterol, or diabetes.
- •
- are pregnant or plan to become pregnant. It is not known if TRIZIVIR will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
Pregnancy Registry. If you take TRIZIVIR while you are pregnant, talk to your healthcare provider about how you can take part in the Pregnancy Registry for TRIZIVIR. The purpose of the pregnancy registry is to collect information about the health of you and your baby.
- •
- are breastfeeding or plan to breastfeed. Do not breastfeed. Lamivudine and zidovudine are excreted in human breast milk. We do not know if abacavir can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- •
- alcohol
- •
- medicines used to treat hepatitis viruses such as interferon or ribavirin
- •
- methadone
- •
- BACTRIM®, SEPTRA® (trimethoprim [TMP/sulfamethoxazole SMX])
- •
- CYTOVENE®, DHPG (ganciclovir)
- •
- interferon-alfa
- •
- ADRIAMYCIN® (doxorubicin)
- •
- COPEGUS®, REBETOL®, VIRAZOLE® (ribavirin)
- •
- any bone marrow suppressive medicines or cytotoxic medicines. Ask your doctor if you are not sure.
- •
- ATRIPLA® (efavirenz/emtricitabine/tenofovir disoproxil fumarate)
- •
- COMBIVIR® (lamivudine and zidovudine)
- •
- COMPLERA® (emtricitabine/rilpivirine/tenofovir disoproxil fumarate)
- •
- EMTRIVA® (emtricitabine)
- •
- EPIVIR or EPIVIR-HBV® (lamivudine)
- •
- EPZICOM (abacavir sulfate and lamivudine)
- •
- RETROVIR (zidovudine)
- •
- TRUVADA® (emtricitabine/tenofovir disoproxil fumarate)
- •
- ZERIT® (stavudine)
- •
- ZIAGEN (abacavir sulfate)
Ask your healthcare provider if you are not sure if you take one of the medicines listed above.
TRIZIVIR may affect the way other medicines work, and other medicines may affect how TRIZIVIR works.
Know the medicines you take. Keep a list of your medicines with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take TRIZIVIR?
- •
- Take TRIZIVIR exactly as your healthcare provider tells you to take it.
- •
- TRIZIVIR may be taken with or without food.
- •
- Do not skip doses.
- •
- Do not let your TRIZIVIR run out.
If you stop your anti-HIV medicines, even for a short time, the amount of virus in your blood may increase and the virus may become harder to treat.If you take too much TRIZIVIR, call your healthcare provider or poison control center or go to the nearest hospital emergency room right away.
What are the possible side effects of TRIZIVIR?
TRIZIVIR can cause serious side effects including allergic reactions, lactic acidosis, and liver problems. See “What is the most important information I should know about TRIZIVIR?”
- •
- Blood problems.
- •
- Muscle weakness.
- •
- Changes in immune system (Immune Reconstitution Syndrome). Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider if you start having new or worse symptoms of infection after you start taking TRIZIVIR.
- •
- Changes in body fat (fat redistribution). Changes in body fat (lipoatrophy or lipodystrophy) can happen in some people taking antiretroviral medicines including TRIZIVIR.
- These changes may include:
- •
- more fat in or around your trunk, upper back and neck (buffalo hump), breast or chest
- •
- loss of fat in your legs, arms, or face
- •
- Heart attack (myocardial infarction). Some HIV medicines including TRIZIVIR may increase your risk of heart attack.
The most common side effects of TRIZIVIR include:
- •
- nausea
- •
- headache
- •
- weakness or tiredness
- •
- vomiting
- •
- diarrhea
- •
- fever and/or chills
- •
- depression
- •
- muscle and joint pain
- •
- skin rashes
- •
- ear, nose, throat infections
- •
- cold symptoms
- •
- nervousness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of TRIZIVIR. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TRIZIVIR?
- •
- Store TRIZIVIR at 59°F to 86°F (15°C to 30°C).
- •
- Keep TRIZIVIR and all medicines out of the reach of children.
General information for safe and effective use of TRIZIVIR.
Avoid doing things that can spread HIV-1 infection to others.
- •
- Do not share needles or other injection equipment.
- •
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- •
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TRIZIVIR for a condition for which it was not prescribed. Do not give TRIZIVIR to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about TRIZIVIR. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for the information about TRIZIVIR that is written for healthcare professionals.
For more information go to www.TRIZIVIR.com or call 1-877-844-8872.
What are the ingredients in TRIZIVIR?
Active ingredients: abacavir sulfate, lamivudine, and zidovudine
Inactive ingredients: magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and OPADRY® green 03B11434, a film coating made of FD&C Blue No. 2, hypromellose, polyethylene glycol, titanium dioxide, and yellow iron oxide.
This Medication Guide has been approved by the US Food and Drug Administration.
COMBIVIR, EPIVIR, EPZICOM, RETROVIR, TRIZIVIR, and ZIAGEN are registered trademarks of ViiV Healthcare.
The brands listed are trademarks of their respective owners and are not trademarks of ViiV Healthcare. The makers of these brands are not affiliated with and do not endorse ViiV Healthcare or its products.
Manufactured for:
ViiV Healthcare
Research Triangle Park, NC 27709
by:
GlaxoSmithKline
Research Triangle Park, NC 27709
Lamivudine is manufactured under agreement from
Shire Pharmaceuticals Group plc
Basingstoke, UK
©2013, ViiV Healthcare. All rights reserved.
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
TRIZIVIR
abacavir sulfate, lamivudine, and zidovudine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53808-0990(NDC:49702-217) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ABACAVIR SULFATE (UNII: J220T4J9Q2) (ABACAVIR - UNII:WR2TIP26VS) ABACAVIR 300 mg LAMIVUDINE (UNII: 2T8Q726O95) (LAMIVUDINE - UNII:2T8Q726O95) LAMIVUDINE 150 mg ZIDOVUDINE (UNII: 4B9XT59T7S) (ZIDOVUDINE - UNII:4B9XT59T7S) ZIDOVUDINE 300 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color BLUE (blue-green) Score no score Shape OVAL (capsule-shaped) Size 21mm Flavor Imprint Code GX;LL1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53808-0990-1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021205 02/01/2013 Labeler - State of Florida DOH Central Pharmacy (829348114) Establishment Name Address ID/FEI Business Operations State of Florida DOH Central Pharmacy 829348114 repack(53808-0990)